Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions
- PMID: 28351383
- PMCID: PMC5370462
- DOI: 10.1186/s13059-017-1185-3
Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions
Abstract
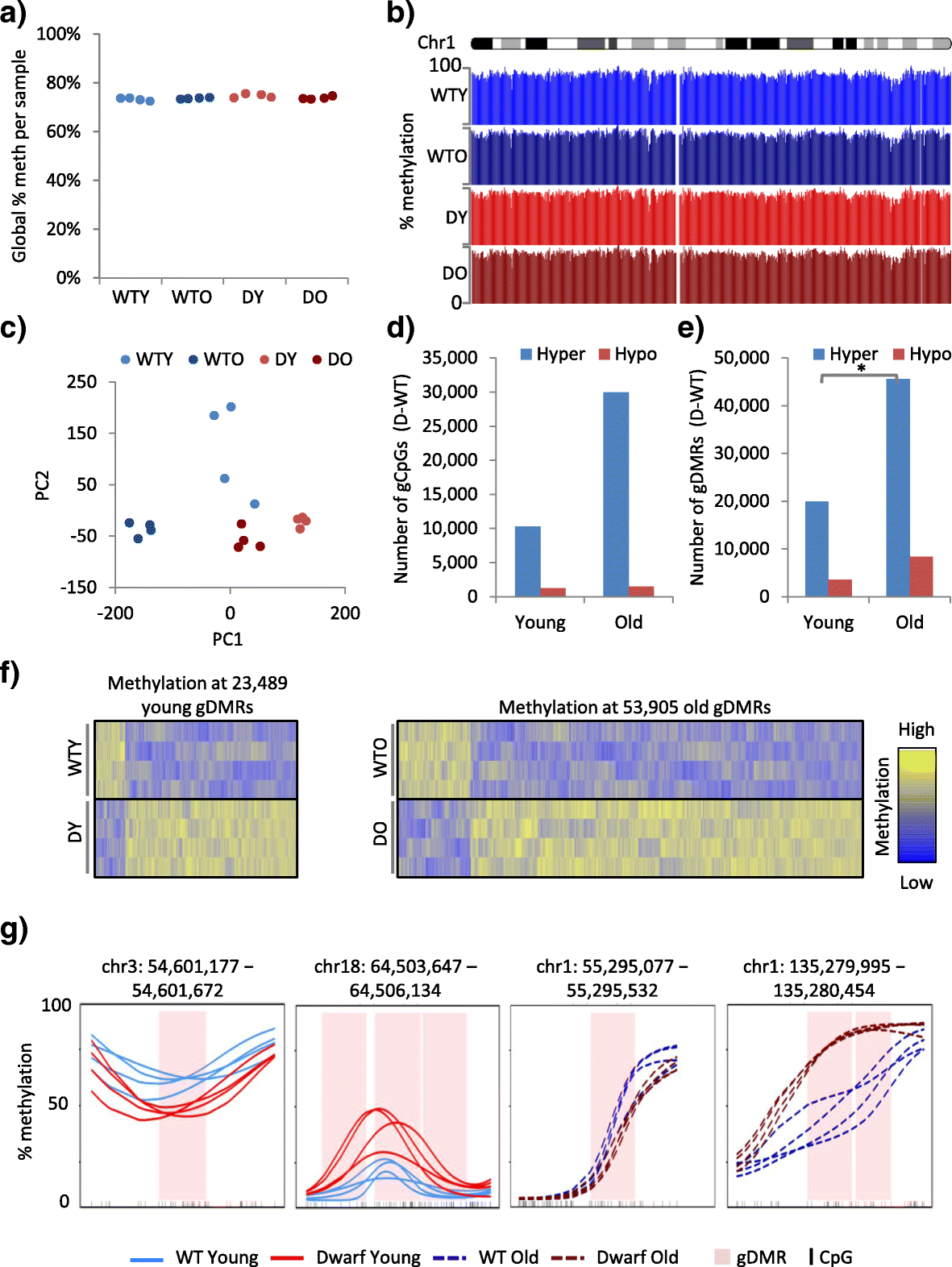
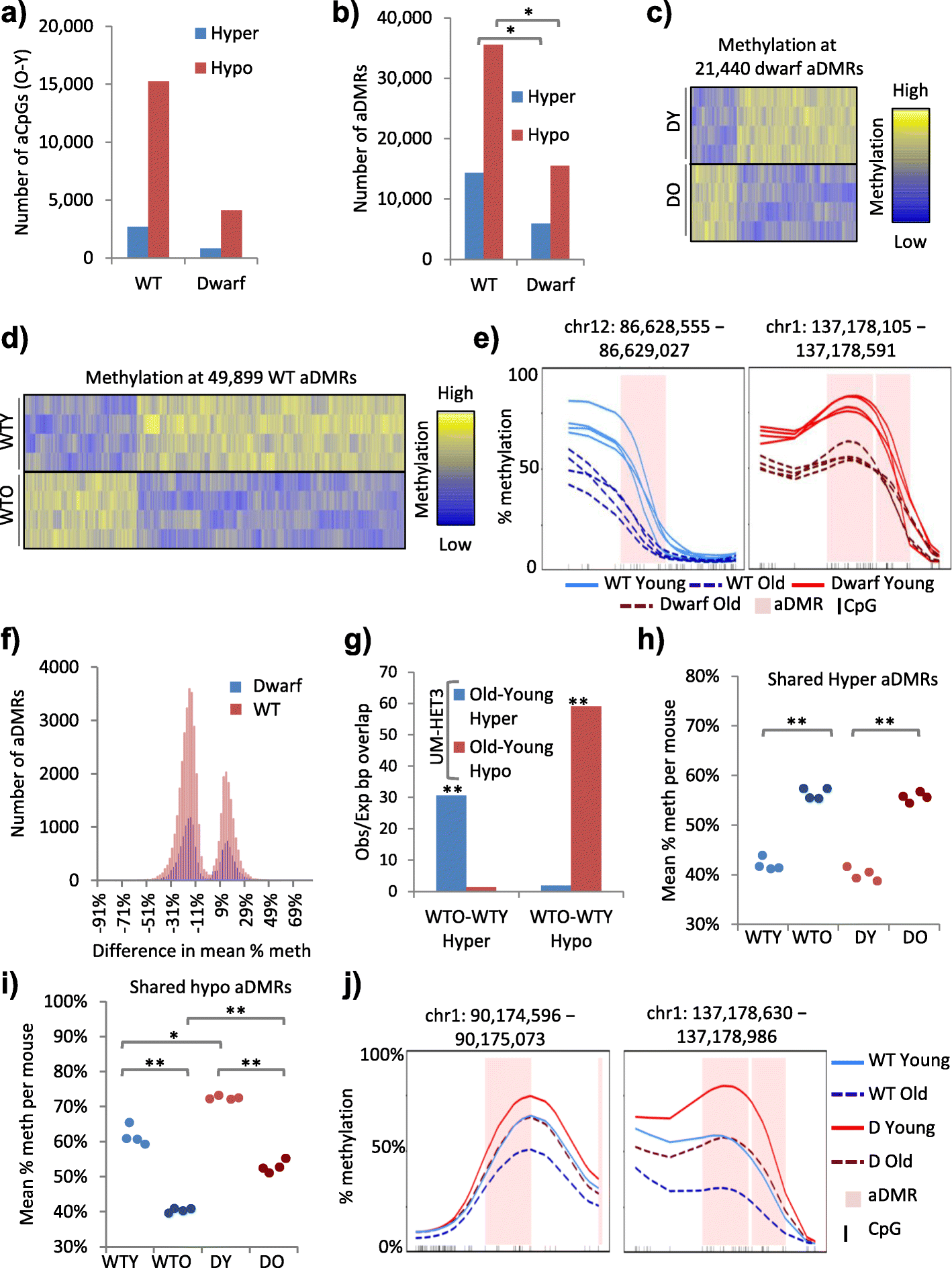
Background: Age-associated epigenetic changes are implicated in aging. Notably, age-associated DNA methylation changes comprise a so-called aging "clock", a robust biomarker of aging. However, while genetic, dietary and drug interventions can extend lifespan, their impact on the epigenome is uncharacterised. To fill this knowledge gap, we defined age-associated DNA methylation changes at the whole-genome, single-nucleotide level in mouse liver and tested the impact of longevity-promoting interventions, specifically the Ames dwarf Prop1 df/df mutation, calorie restriction and rapamycin.
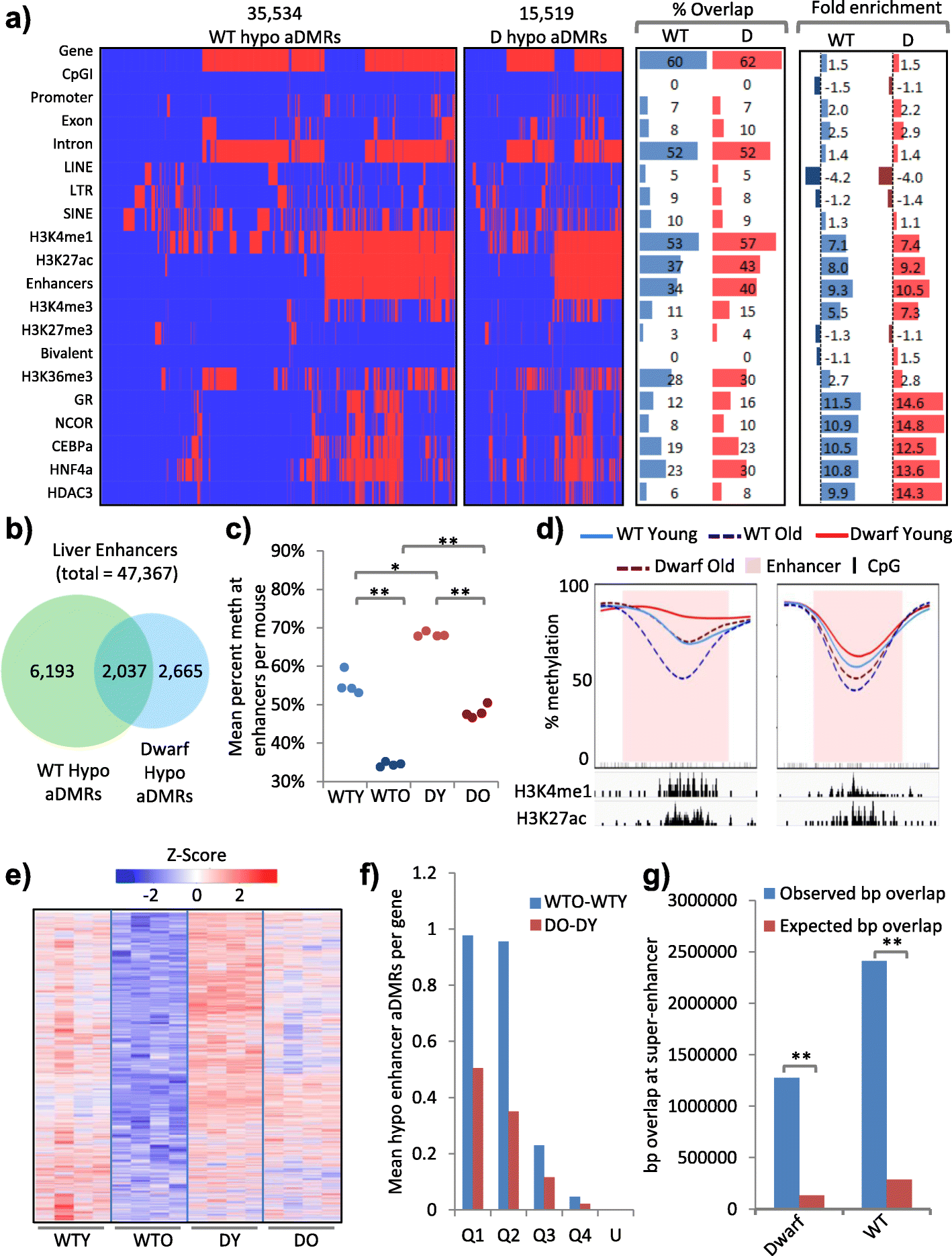
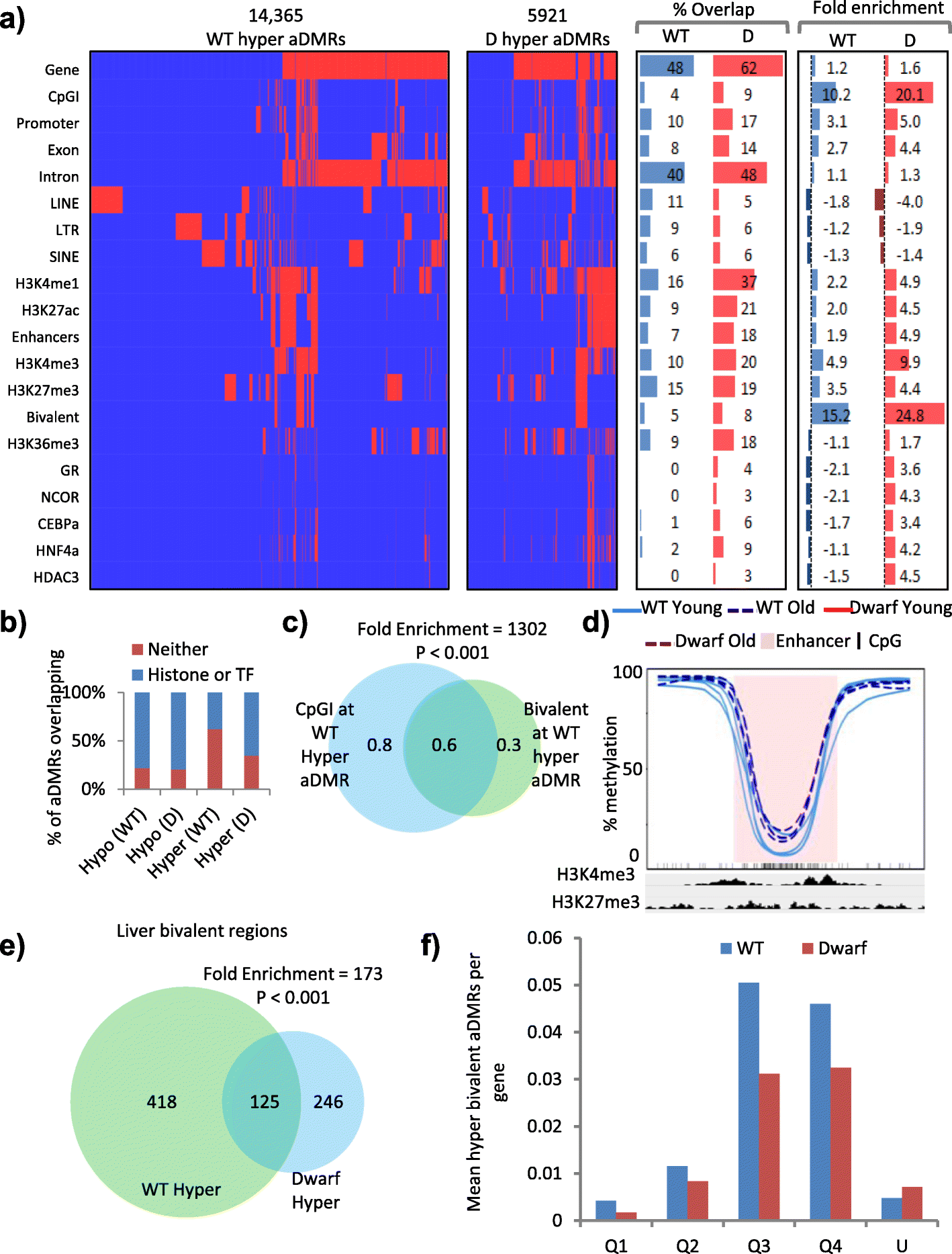
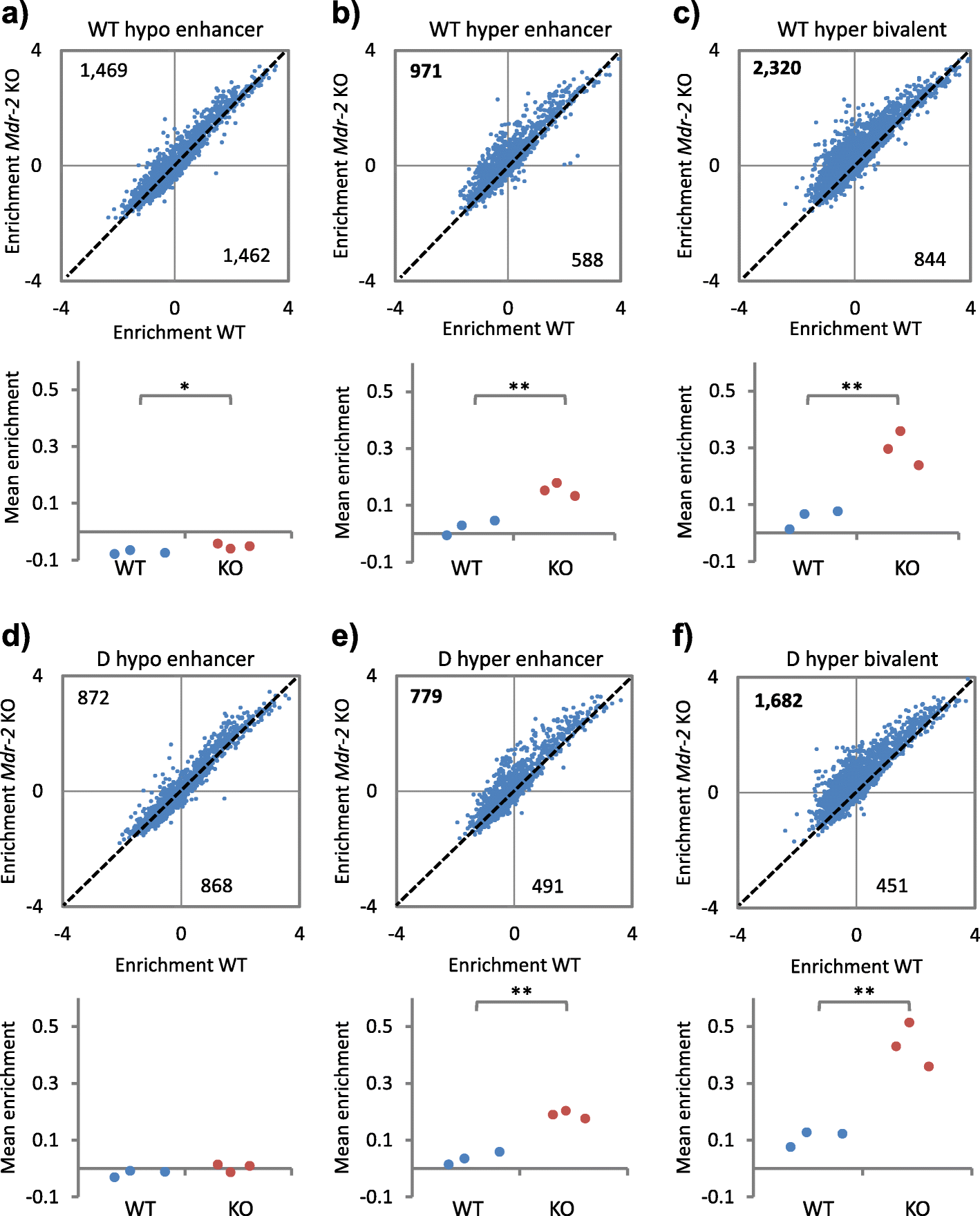
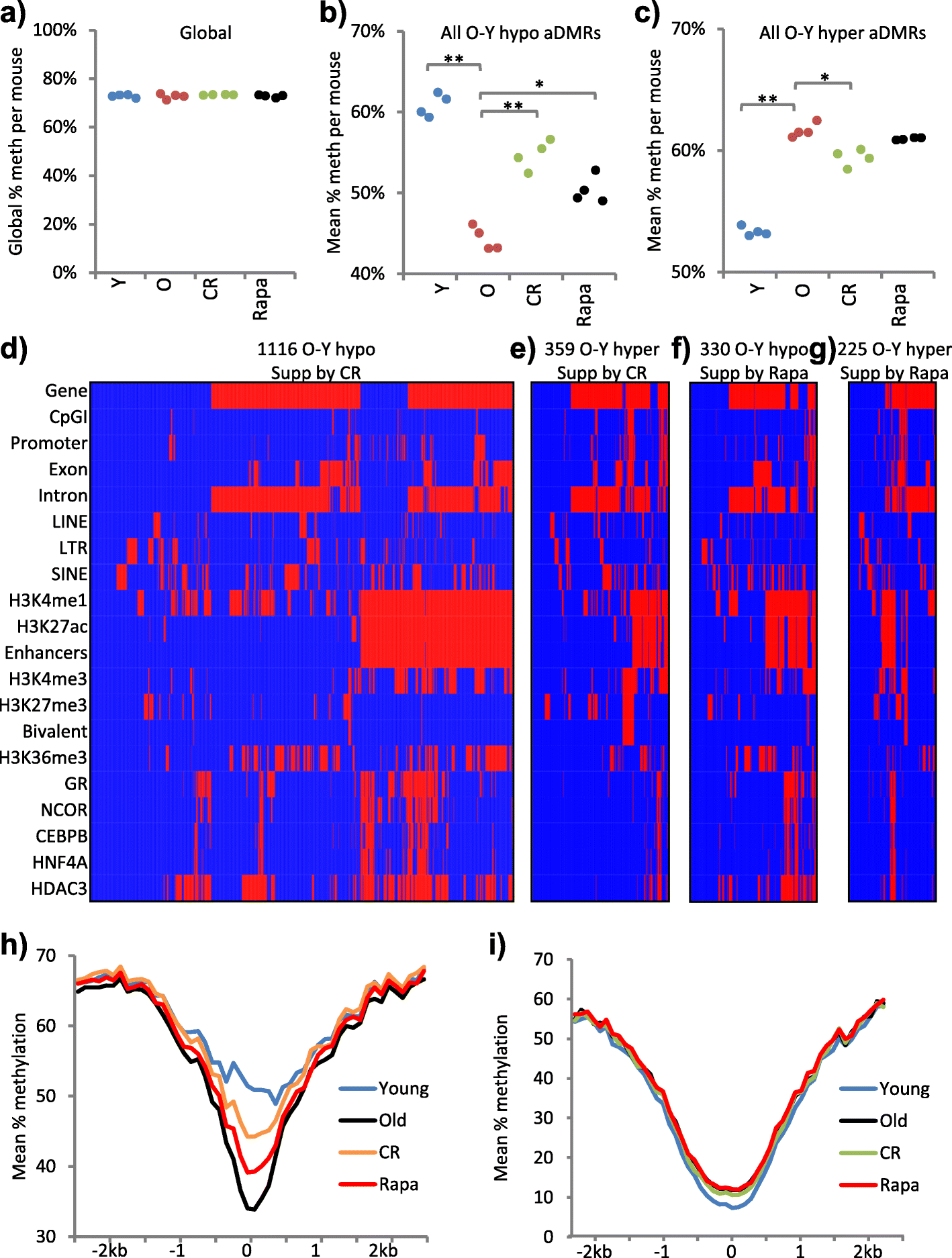
Results: In wild-type mice fed an unsupplemented ad libitum diet, age-associated hypomethylation was enriched at super-enhancers in highly expressed genes critical for liver function. Genes harbouring hypomethylated enhancers were enriched for genes that change expression with age. Hypermethylation was enriched at CpG islands marked with bivalent activating and repressing histone modifications and resembled hypermethylation in liver cancer. Age-associated methylation changes are suppressed in Ames dwarf and calorie restricted mice and more selectively and less specifically in rapamycin treated mice.
Conclusions: Age-associated hypo- and hypermethylation events occur at distinct regulatory features of the genome. Distinct longevity-promoting interventions, specifically genetic, dietary and drug interventions, suppress some age-associated methylation changes, consistent with the idea that these interventions exert their beneficial effects, in part, by modulation of the epigenome. This study is a foundation to understand the epigenetic contribution to healthy aging and longevity and the molecular basis of the DNA methylation clock.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

