Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism
- PMID: 28351387
- PMCID: PMC5370449
- DOI: 10.1186/s13059-017-1187-1
Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism
Abstract
Background: Dietary restriction (DR), a reduction in food intake without malnutrition, increases most aspects of health during aging and extends lifespan in diverse species, including rodents. However, the mechanisms by which DR interacts with the aging process to improve health in old age are poorly understood. DNA methylation could play an important role in mediating the effects of DR because it is sensitive to the effects of nutrition and can affect gene expression memory over time.
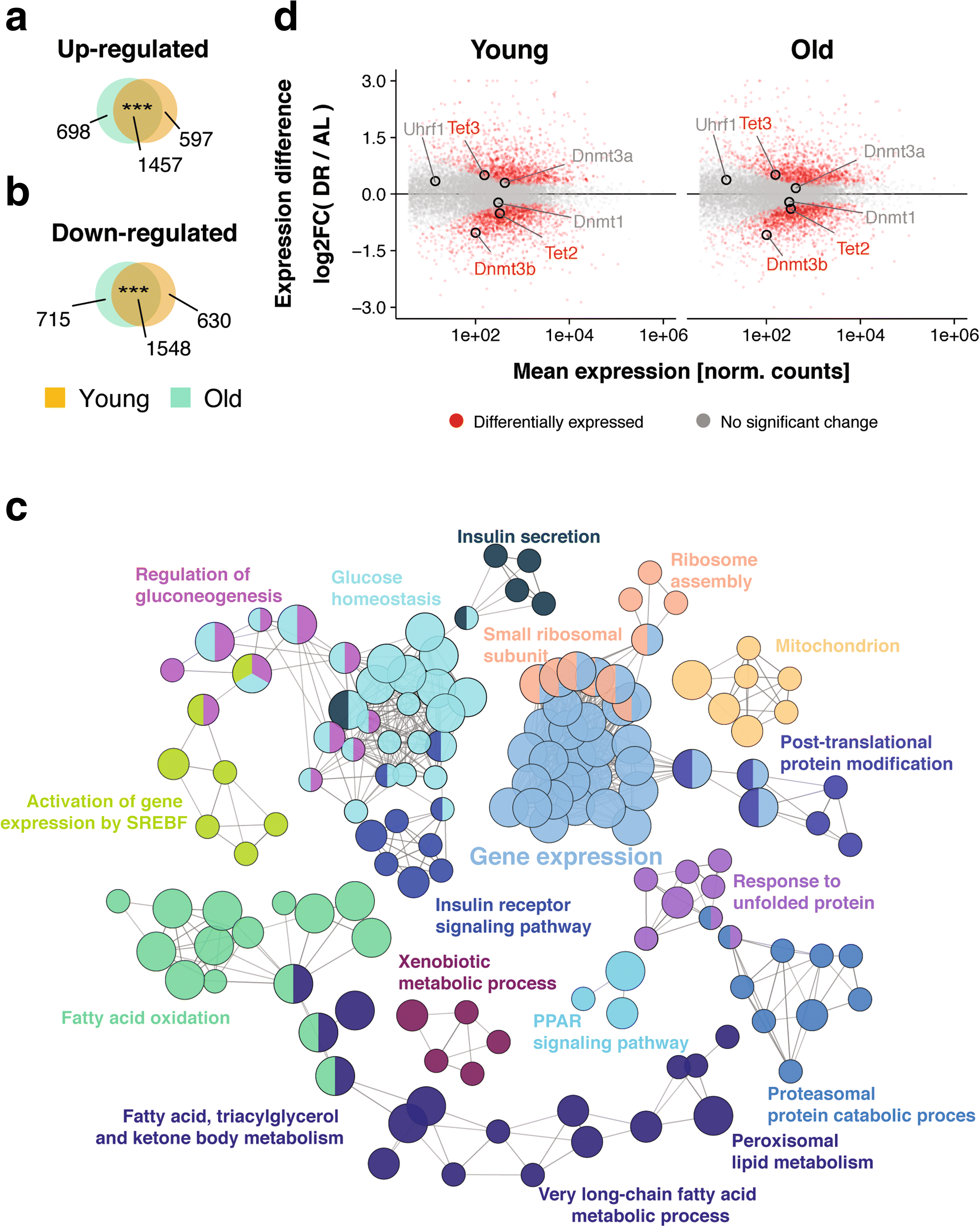
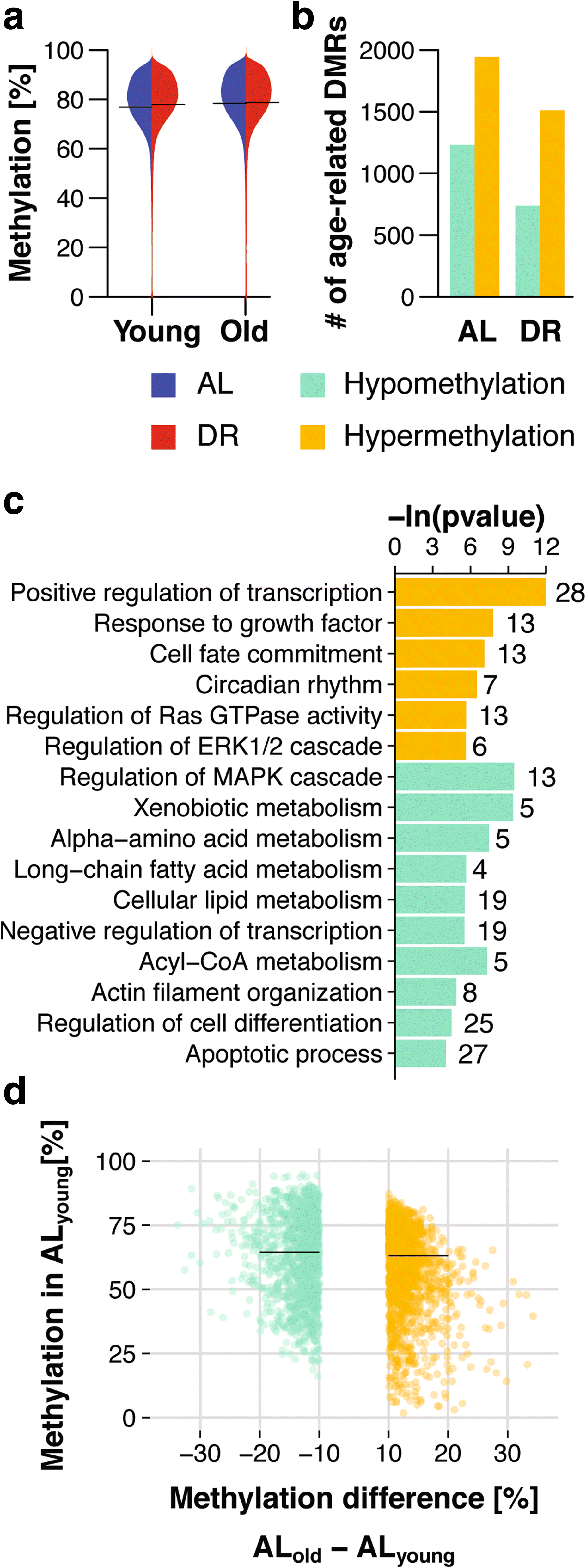
Results: Here, we profile genome-wide changes in DNA methylation, gene expression and lipidomics in response to DR and aging in female mouse liver. DR is generally strongly protective against age-related changes in DNA methylation. During aging with DR, DNA methylation becomes targeted to gene bodies and is associated with reduced gene expression, particularly of genes involved in lipid metabolism. The lipid profile of the livers of DR mice is correspondingly shifted towards lowered triglyceride content and shorter chain length of triglyceride-associated fatty acids, and these effects become more pronounced with age.
Conclusions: Our results indicate that DR remodels genome-wide patterns of DNA methylation so that age-related changes are profoundly delayed, while changes at loci involved in lipid metabolism affect gene expression and the resulting lipid profile.
Figures




References
-
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/M004821/1/MRC_/Medical Research Council/United Kingdom
- 268739/ERC_/European Research Council/International
- WT_/Wellcome Trust/United Kingdom
- BBS/E/B/000C0417/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

