Targeting of 12/15-Lipoxygenase in retinal endothelial cells, but not in monocytes/macrophages, attenuates high glucose-induced retinal leukostasis
- PMID: 28351645
- PMCID: PMC5504079
- DOI: 10.1016/j.bbalip.2017.03.010
Targeting of 12/15-Lipoxygenase in retinal endothelial cells, but not in monocytes/macrophages, attenuates high glucose-induced retinal leukostasis
Abstract
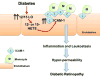
Aims: Our previous studies have established a role for 12/15-lipoxygenase (LO) in mediating the inflammatory response in diabetic retinopathy (DR). However, the extent at which the local or systemic induction of 12/15-LO activity involved is unclear. Thus, the current study aimed to characterize the relative contribution of retinal endothelial versus monocytic/macrophagic 12/15-LO to inflammatory responses in DR.
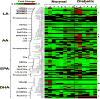
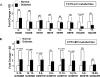
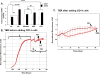
Materials & methods: We first generated a clustered heat map for circulating bioactive lipid metabolites in the plasma of streptozotocin (STZ)-induced diabetic mice using liquid chromatography coupled with mass-spectrometry (LC-MS) to evaluate changes in circulating 12/15-LO activity. This was followed by comparing the in vitro mouse endothelium-leukocytes interaction between leukocytes isolated from 12/15-LO knockout (KO) versus those isolated from wild type (WT) mice using the myeloperoxidase (MPO) assay. Finally, we examined the effects of knocking down or inhibiting endothelial 12/15-LO on diabetes-induced endothelial cell activation and ICAM-1 expression.
Results: Analysis of plasma bioactive lipids' heat map revealed that the activity of circulating 12/15-LO was not altered by diabetes as evident by no significant changes in the plasma levels of major metabolites derived from 12/15-lipoxygenation of different PUFAs, including linoleic acid (13-HODE), arachidonic acid (12- and 15- HETEs), eicosapentaenoic acid (12- and 15- HEPEs), or docosahexaenoic acid (17-HDoHE). Moreover, leukocytes from 12/15-LO KO mice displayed a similar increase in adhesion to high glucose (HG)-activated endothelial cells as do leukocytes from WT mice. Furthermore, abundant proteins of 12-LO and 15-LO were detected in human retinal endothelial cells (HRECs), while it was undetected (15-LO) or hardly detectable (12-LO) in human monocyte-like U937 cells. Inhibition or knock down of endothelial 12/15-LO in HRECs blocked HG-induced expression of ICAM-1, a well-known identified important molecule for leukocyte adhesion in DR.
Conclusion: Our data support that endothelial, rather than monocytic/macrophagic, 12/15-LO has a critical role in hyperglycemia-induced ICAM-1 expression, leukocyte adhesion, and subsequent local retinal barrier dysfunction. This may facilitate the development of more precisely targeted treatment strategies for DR.
Keywords: 12-HETE; 12/15-Lipoxygenase; 15-HETE; Bioactive lipids; Blood retinal barrier; Diabetic retinopathy; Eicosanoids; ICAM-1; Leukostasis; Permeability.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy.Diabetologia. 2018 May;61(5):1220-1232. doi: 10.1007/s00125-018-4560-z. Epub 2018 Feb 21. Diabetologia. 2018. PMID: 29468369 Free PMC article.
-
Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids.Biochim Biophys Acta. 2015 Mar;1851(3):290-8. doi: 10.1016/j.bbalip.2014.12.017. Epub 2015 Jan 3. Biochim Biophys Acta. 2015. PMID: 25562624 Free PMC article.
-
A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase.J Lipid Res. 2015 Mar;56(3):599-611. doi: 10.1194/jlr.M056069. Epub 2015 Jan 17. J Lipid Res. 2015. PMID: 25598081 Free PMC article.
-
Key Role of 12-Lipoxygenase and Its Metabolite 12-Hydroxyeicosatetraenoic Acid (12-HETE) in Diabetic Retinopathy.Curr Eye Res. 2022 Mar;47(3):329-335. doi: 10.1080/02713683.2021.1995003. Epub 2022 Feb 7. Curr Eye Res. 2022. PMID: 35129022 Review.
-
Is leukostasis a crucial step or epiphenomenon in the pathogenesis of diabetic retinopathy?J Leukoc Biol. 2017 Oct;102(4):993-1001. doi: 10.1189/jlb.3RU0417-139. Epub 2017 Jul 19. J Leukoc Biol. 2017. PMID: 28724696 Review.
Cited by
-
Role of the 12-lipoxygenase pathway in diabetes pathogenesis and complications.Pharmacol Ther. 2019 Mar;195:100-110. doi: 10.1016/j.pharmthera.2018.10.010. Epub 2018 Oct 19. Pharmacol Ther. 2019. PMID: 30347209 Free PMC article. Review.
-
Bioactive lipids and pathological retinal angiogenesis.Br J Pharmacol. 2019 Jan;176(1):93-109. doi: 10.1111/bph.14507. Epub 2018 Nov 19. Br J Pharmacol. 2019. PMID: 30276789 Free PMC article. Review.
-
WFDC12-overexpressing contributes to the development of atopic dermatitis via accelerating ALOX12/15 metabolism and PAF accumulation.Cell Death Dis. 2023 Mar 8;14(3):185. doi: 10.1038/s41419-023-05686-3. Cell Death Dis. 2023. PMID: 36882395 Free PMC article.
-
Possible Role of Endothelial-Derived Cellular and Exosomal-miRNAs in Lipid-Mediated Diabetic Retinopathy: Microarray Studies.Cells. 2024 Nov 15;13(22):1886. doi: 10.3390/cells13221886. Cells. 2024. PMID: 39594634 Free PMC article.
-
Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy.Diabetologia. 2018 May;61(5):1220-1232. doi: 10.1007/s00125-018-4560-z. Epub 2018 Feb 21. Diabetologia. 2018. PMID: 29468369 Free PMC article.
References
-
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. - PubMed
-
- Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE, Cotch MF, Wong TY. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31:544–549. - PubMed
-
- Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, G Global Diabetic Retinopathy Project Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. - PubMed
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

