Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma
- PMID: 28351668
- PMCID: PMC5636223
- DOI: 10.1016/j.jconrel.2017.03.384
Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma
Abstract
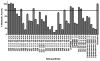
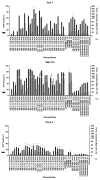
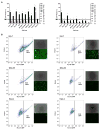
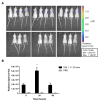
Hepatocellular carcinoma (HCC) is the third most deadly cancer in the US, with a meager 5-year survival rate of <20%. Such unfavorable numbers are closely related to the heterogeneity of the disease and the unsatisfactory therapies currently used to manage patients with invasive HCC. Outside of the clinic, gene therapy research is evolving to overcome the poor responses and toxicity associated with standard treatments. The inadequacy of gene delivery vectors, including poor intracellular delivery and cell specificity, are major barriers in the gene therapy field. Herein, we described a non-viral strategy for effective and cancer-specific DNA delivery to human HCC using biodegradable poly(beta-amino ester) (PBAE) nanoparticles (NPs). Varied PBAE NP formulations were evaluated for transfection efficacy and cytotoxicity to a range of human HCC cells as well as healthy human hepatocytes. To address HCC heterogeneity, nine different sources of human HCC cells were utilized. The polymeric NPs composed of 2-((3-aminopropyl)amino) ethanol end-modified poly(1,5-pentanediol diacrylate-co-3-amino-1-propanol) ('536') at a 25 polymer-to-DNA weight-to-weight ratio led to high transfection efficacy to all of the liver cancer lines, but not to hepatocytes. Each individual HCC line had a significantly higher percentage of exogenous gene expression than the healthy liver cells (P<0.01). Notably, this biodegradable end-modified PBAE gene delivery vector was not cytotoxic and maintained the viability of hepatocytes above 80%. In a HCC/hepatocyte co-culture model, in which cancerous and healthy cells share the same micro-environment, 536 25 w/w NPs specifically transfected cancer cells. PBAE NP administration to a subcutaneous HCC mouse model, established with one of the human lines tested in vitro, confirmed effective DNA transfection in vivo. PBAE-based NPs enabled high and preferential DNA delivery to HCC cells, sparing healthy hepatocytes. These biodegradable and liver cancer-selective NPs are a promising technology to deliver therapeutic genes to liver cancer.
Keywords: Carcinoma, hepatocellular; Gene therapy; Nanoparticle.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

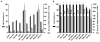


References
-
- El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Hashem BES. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. - PubMed
-
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L. Surveillance, Epidemiology, and End Results (SEER) Program SEER* Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002) National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; 2005.
-
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

