Elucidating transmission dynamics and host-parasite-vector relationships for rodent-borne Bartonella spp. in Madagascar
- PMID: 28351673
- PMCID: PMC5608689
- DOI: 10.1016/j.epidem.2017.03.004
Elucidating transmission dynamics and host-parasite-vector relationships for rodent-borne Bartonella spp. in Madagascar
Abstract
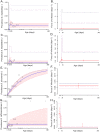
Bartonella spp. are erythrocytic bacteria transmitted via arthropod vectors, which infect a broad range of vertebrate hosts, including humans. We investigated transmission dynamics and host-parasite-vector relationships for potentially zoonotic Bartonella spp. in invasive Rattus rattus hosts and associated arthropod ectoparasites in Madagascar. We identified five distinct species of Bartonella (B. elizabethae 1, B. elizabethae 2, B. phoceensis 1, B. rattimassiliensis 1, and B. tribocorum 1) infecting R. rattus rodents and their ectoparasites. We fit standard epidemiological models to species-specific age-prevalence data for the four Bartonella spp. with sufficient data, thus quantifying age-structured force of infection. Known zoonotic agents, B. elizabethae 1 and 2, were best described by models exhibiting high forces of infection in early age class individuals and allowing for recovery from infection, while B. phoceensis 1 and B. rattimassiliensis 1 were best fit by models of lifelong infection without recovery and substantially lower forces of infection. Nested sequences of B. elizabethae 1 and 2 were recovered from rodent hosts and their Synopsyllus fonquerniei and Xenopsylla cheopsis fleas, with a particularly high prevalence in the outdoor-dwelling, highland-endemic S. fonquerniei. These findings expand on force of infection analyses to elucidate the ecological niche of the zoonotic Bartonella elizabethae complex in Madagascar, hinting at a potential vector role for S. fonquerniei. Our analyses underscore the uniqueness of such ecologies for Bartonella species, which pose a variable range of potential zoonotic threats.
Keywords: Bartonella spp.; Force of infection; Madagascar; Rattus rattus; Synopsyllus fonquerniei.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Bai Y., Kosoy M.Y., Ray C., Brinkerhoff R.J., Collinge S.K. Temporal and spatial patterns of Bartonella infection in black-tailed prairie dogs (Cynomys ludovicianus) Microb. Ecol. 2008;56:373–382. - PubMed
-
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48.
-
- Battisttini T. Verruga peruana: Sa transmission par le phlébotome. Rev. Sud-Americaine Med. Chir. 1931;2:719–724.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

