Cyclin C regulates adipogenesis by stimulating transcriptional activity of CCAAT/enhancer-binding protein α
- PMID: 28351837
- PMCID: PMC5448125
- DOI: 10.1074/jbc.M117.776229
Cyclin C regulates adipogenesis by stimulating transcriptional activity of CCAAT/enhancer-binding protein α
Abstract
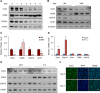
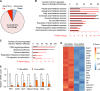
Brown adipose tissue is important for maintaining energy homeostasis and adaptive thermogenesis in rodents and humans. As disorders arising from dysregulated energy metabolism, such as obesity and metabolic diseases, have increased, so has interest in the molecular mechanisms of adipocyte biology. Using a functional screen, we identified cyclin C (CycC), a conserved subunit of the Mediator complex, as a novel regulator for brown adipocyte formation. siRNA-mediated CycC knockdown (KD) in brown preadipocytes impaired the early transcriptional program of differentiation, and genetic KO of CycC completely blocked the differentiation process. RNA sequencing analyses of CycC-KD revealed a critical role of CycC in activating genes co-regulated by peroxisome proliferator activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα). Overexpression of PPARγ2 or addition of the PPARγ ligand rosiglitazone rescued the defects in CycC-KO brown preadipocytes and efficiently activated the PPARγ-responsive promoters in both WT and CycC-KO cells, suggesting that CycC is not essential for PPARγ transcriptional activity. In contrast, CycC-KO significantly reduced C/EBPα-dependent gene expression. Unlike for PPARγ, overexpression of C/EBPα could not induce C/EBPα target gene expression in CycC-KO cells or rescue the CycC-KO defects in brown adipogenesis, suggesting that CycC is essential for C/EBPα-mediated gene activation. CycC physically interacted with C/EBPα, and this interaction was required for C/EBPα transactivation domain activity. Consistent with the role of C/EBPα in white adipogenesis, CycC-KD also inhibited differentiation of 3T3-L1 cells into white adipocytes. Together, these data indicate that CycC activates adipogenesis in part by stimulating the transcriptional activity of C/EBPα.
Keywords: C/EBPα; Mediator complex; PPARγ; adipocyte; adipogenesis; brown adipocyte; cell differentiation; cyclin C; gene expression; transcription coregulator.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Krüppel-like factor 10 (KLF10) is transactivated by the transcription factor C/EBPβ and involved in early 3T3-L1 preadipocyte differentiation.J Biol Chem. 2018 Sep 7;293(36):14012-14021. doi: 10.1074/jbc.RA118.004401. Epub 2018 Jul 19. J Biol Chem. 2018. PMID: 30026232 Free PMC article.
-
A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes.J Biol Chem. 2001 May 25;276(21):18464-71. doi: 10.1074/jbc.M100797200. Epub 2001 Feb 27. J Biol Chem. 2001. PMID: 11279134
-
Histone H3K9 Demethylase JMJD2B Activates Adipogenesis by Regulating H3K9 Methylation on PPARγ and C/EBPα during Adipogenesis.PLoS One. 2017 Jan 6;12(1):e0168185. doi: 10.1371/journal.pone.0168185. eCollection 2017. PLoS One. 2017. PMID: 28060835 Free PMC article.
-
Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β.J Biol Chem. 2015 Jan 9;290(2):755-61. doi: 10.1074/jbc.R114.619957. Epub 2014 Dec 1. J Biol Chem. 2015. PMID: 25451943 Free PMC article. Review.
-
The contribution of vitamin A to autocrine regulation of fat depots.Biochim Biophys Acta. 2012 Jan;1821(1):190-7. doi: 10.1016/j.bbalip.2011.06.004. Epub 2011 Jun 13. Biochim Biophys Acta. 2012. PMID: 21704731 Free PMC article. Review.
Cited by
-
Activation of skeletal carbohydrate-response element binding protein (ChREBP)-mediated de novo lipogenesis increases intramuscular fat content in chickens.Anim Nutr. 2024 Apr 16;18:107-118. doi: 10.1016/j.aninu.2024.04.006. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39091296 Free PMC article.
-
A novel brown adipocyte-enriched long non-coding RNA that is required for brown adipocyte differentiation and sufficient to drive thermogenic gene program in white adipocytes.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Apr;1863(4):409-419. doi: 10.1016/j.bbalip.2018.01.008. Epub 2018 Jan 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29341928 Free PMC article.
-
Effects of Opuntia stricta var. dillenii Extracts Obtained from Prickly Pear and an Industrial By-Product on Maturing Pre-Adipocytes.Plants (Basel). 2024 Oct 24;13(21):2967. doi: 10.3390/plants13212967. Plants (Basel). 2024. PMID: 39519886 Free PMC article.
-
Endocrine regulation of metabolic crosstalk between liver and brown adipose tissue by natural active ingredients.Int J Obes (Lond). 2025 May 19. doi: 10.1038/s41366-025-01793-7. Online ahead of print. Int J Obes (Lond). 2025. PMID: 40389647 Review.
-
MED12 Regulates Human Adipose-Derived Stem Cell Adipogenesis and Mediator Kinase Subunit Expression in Murine Adipose Depots.Stem Cells Dev. 2022 Mar;31(5-6):119-131. doi: 10.1089/scd.2021.0302. Epub 2022 Jan 28. Stem Cells Dev. 2022. PMID: 35018809 Free PMC article.
References
-
- Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., and Flier J. S. (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

