Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity
- PMID: 28351984
- PMCID: PMC5379978
- DOI: 10.1084/jem.20161382
Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity
Abstract
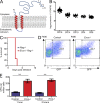
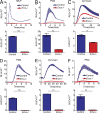
The phagocyte respiratory burst is crucial for innate immunity. The transfer of electrons to oxygen is mediated by a membrane-bound heterodimer, comprising gp91phox and p22phox subunits. Deficiency of either subunit leads to severe immunodeficiency. We describe Eros (essential for reactive oxygen species), a protein encoded by the previously undefined mouse gene bc017643, and show that it is essential for host defense via the phagocyte NAPDH oxidase. Eros is required for expression of the NADPH oxidase components, gp91phox and p22phox Consequently, Eros-deficient mice quickly succumb to infection. Eros also contributes to the formation of neutrophil extracellular traps (NETS) and impacts on the immune response to melanoma metastases. Eros is an ortholog of the plant protein Ycf4, which is necessary for expression of proteins of the photosynthetic photosystem 1 complex, itself also an NADPH oxio-reductase. We thus describe the key role of the previously uncharacterized protein Eros in host defense.
© 2017 Thomas et al.
Figures





References
-
- Ayadi A., Birling M.C., Bottomley J., Bussell J., Fuchs H., Fray M., Gailus-Durner V., Greenaway S., Houghton R., Karp N., et al. . 2012. Mouse large-scale phenotyping initiatives: overview of the European Mouse Disease Clinic (EUMODIC) and of the Wellcome Trust Sanger Institute Mouse Genetics Project. Mamm. Genome. 23:600–610. 10.1007/s00335-012-9418-y - DOI - PMC - PubMed
-
- Berendes H., Bridges R.A., and Good R.A.. 1957. A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn. Med. 40:309–312. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

