Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside
- PMID: 28352078
- PMCID: PMC5428655
- DOI: 10.1038/s41598-017-00568-z
Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside
Erratum in
-
Author Correction: Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside.Sci Rep. 2017 Dec 4;7(1):17147. doi: 10.1038/s41598-017-16990-2. Sci Rep. 2017. PMID: 29203819 Free PMC article.
Abstract
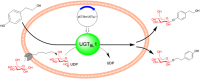
The plant kingdom provides a large resource of natural products and various related enzymes are analyzed. The high catalytic activity and easy genetically modification of microbial enzymes would be beneficial for synthesis of natural products. But the identification of functional genes of target enzymes is time consuming and hampered by many contingencies. The potential to mine microbe-derived glycosyltransferases (GTs) cross the plant kingdom was assessed based on alignment and evolution of the full sequences and key motifs of target enzymes, such as Rhodiola-derived UDP-glycosyltransferase (UGT73B6) using in salidroside synthesis. The GTs from Bacillus licheniformis ZSP01 with high PSPG motif similarity were speculated to catalyze the synthesis of salidroside. The UGTBL1, which had similarity (61.4%) PSPG motif to UGT73B6, displayed efficient activity and similar regioselectivity. Highly efficient glycosylation of tyrosol (1 g/L) was obtained by using engineered E. coli harboring UGTBL1 gene, which generated 1.04 g/L salidroside and 0.99 g/L icariside D2. All glycosides were secreted into the culture medium and beneficial for downstream purification. It was the first report on the genome mining of UGTs from microorganisms cross the plant kingdom. The mining approach may have broader applications in the selection of efficient candidate for making high-value natural products.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Production of salidroside in metabolically engineered Escherichia coli.Sci Rep. 2014 Oct 17;4:6640. doi: 10.1038/srep06640. Sci Rep. 2014. PMID: 25323006 Free PMC article.
-
Molecular cloning and overexpression of a novel UDP-glucosyltransferase elevating salidroside levels in Rhodiola sachalinensis.Plant Cell Rep. 2007 Jul;26(7):989-99. doi: 10.1007/s00299-007-0317-8. Epub 2007 Mar 1. Plant Cell Rep. 2007. PMID: 17333022
-
Mutability landscape guided engineering of a promiscuous microbial glycosyltransferase for regioselective synthesis of salidroside and icariside D2.Int J Biol Macromol. 2024 Apr;263(Pt 1):130229. doi: 10.1016/j.ijbiomac.2024.130229. Epub 2024 Feb 18. Int J Biol Macromol. 2024. PMID: 38378110
-
Insights into functional divergence, catalytic versatility and specificity of small molecule glycosyltransferases.Int J Biol Macromol. 2025 Mar;292:138821. doi: 10.1016/j.ijbiomac.2024.138821. Epub 2024 Dec 19. Int J Biol Macromol. 2025. PMID: 39708858 Review.
-
Multi-strategy ugt mining, modification and glycosyl donor synthesis facilitate the production of triterpenoid saponins.Front Plant Sci. 2025 May 30;16:1586295. doi: 10.3389/fpls.2025.1586295. eCollection 2025. Front Plant Sci. 2025. PMID: 40519597 Free PMC article. Review.
Cited by
-
The sugar donor specificity of plant family 1 glycosyltransferases.Front Bioeng Biotechnol. 2024 May 2;12:1396268. doi: 10.3389/fbioe.2024.1396268. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38756413 Free PMC article. Review.
-
Advances in steroidal saponins biosynthesis.Planta. 2021 Oct 6;254(5):91. doi: 10.1007/s00425-021-03732-y. Planta. 2021. PMID: 34617240 Review.
-
Highly efficient biosynthesis of salidroside by a UDP-glucosyltransferase-catalyzed cascade reaction.Biotechnol Lett. 2024 Apr;46(2):173-181. doi: 10.1007/s10529-023-03453-0. Epub 2024 Jan 6. Biotechnol Lett. 2024. PMID: 38184486
-
A thermostable glycosyltransferase from Paenibacillus polymyxa NJPI29: recombinant expression, characterization, and application in synthesis of glycosides.3 Biotech. 2021 Jul;11(7):314. doi: 10.1007/s13205-021-02855-z. Epub 2021 Jun 4. 3 Biotech. 2021. PMID: 34109099 Free PMC article.
-
ATP binding cassette transporters and uridine diphosphate glycosyltransferases are ancient protein families that evolved roles in herbicide resistance through exaptation.PLoS One. 2023 Sep 21;18(9):e0287356. doi: 10.1371/journal.pone.0287356. eCollection 2023. PLoS One. 2023. PMID: 37733747 Free PMC article.
References
-
- Ju YH, Clausen LM, Allred KF, Almada AL, Helferich WG. β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. The Journal of nutrition. 2004;134:1145–1151. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

