Recruitment of Lyn from endomembranes to the plasma membrane through calcium-dependent cell-cell interactions upon polarization of inducible Lyn-expressing MDCK cells
- PMID: 28352128
- PMCID: PMC5428707
- DOI: 10.1038/s41598-017-00538-5
Recruitment of Lyn from endomembranes to the plasma membrane through calcium-dependent cell-cell interactions upon polarization of inducible Lyn-expressing MDCK cells
Abstract
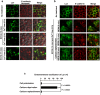
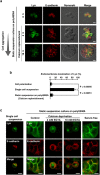
Src-family kinases, expressed in a wide variety of cell types, are anchored to cellular membranes through posttranslational lipid modifications and involved in diverse cellular signalling. In epithelial cells, Src-family kinases are localized at the plasma membrane and participate in epithelial functions. Epithelial cell polarity is achieved through dynamic reorganization of protein trafficking. To examine the trafficking of Src-family kinases between polarized and non-polarized epithelial cells, we generated an MDCK cell line that can inducibly express a protein of interest in a polarized state at any time. We show here that Lyn, a member of Src-family kinases, mainly localizes to the plasma membrane in polarized MDCK cells and to endomembranes in non-polarized MDCK cells. Cell-cell interactions between adjacent MDCK cells recruit Lyn from endomembranes to the plasma membrane even without cell attachment to extracellular matrix scaffolds, and loss of cell-cell interactions by calcium deprivation relocates Lyn from the plasma membrane to endomembranes through Rab11-mediated recycling. Therefore, using our MDCK cells expressing inducible Lyn, we reveal that calcium-dependent cell-cell interactions play a critical role in plasma membrane localization of Lyn in polarized MDCK cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1996;1287:121–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

