HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo
- PMID: 28352154
- PMCID: PMC5359008
- DOI: 10.2147/DDDT.S129913
HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo
Abstract
Background: Diabetic retinopathy (DR), one of the most common complications of late-phase diabetes, is associated with many risk factors, among which continuous low-grade inflammation is one of the principal ones. As such, lowering inflammation levels and maintain the viability of human retinal endothelial cells (HRECs) are critical for DR therapy. HMGB1 is a well-known proinflammatory cytokine. However, whether HMGB1 small interfering RNA (siRNA) can protect retina cells under a high-glucose environment from morphological changes and functional abnormalities remain undetermined. We aimed to investigate the effect of HMGB1 siRNA on retinal cells in DR.
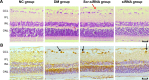
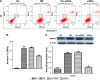
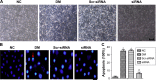
Materials and methods: A total of 80 adult Wistar rats were randomly divided into four groups (n=20 each): normal control, diabetes mellitus (DM), scrambled (Scr) siRNA, and HMGB1 siRNA. Rats in the DM, Scr siRNA, and siRNA groups were established by intraperitoneal injection of streptozotocin. At 16 weeks after injection, rats in the siRNA and Scr-siRNA groups were intravitreally injected with 2 μL HMGB1 siRNA and 2 μL Scr-siRNA, while rats in the control and DM groups were intravitreally injected with the same dose of sterile saline. At 1 week after injections, we performed the following experiments. Immunohistochemical staining and real-time quantitative polymerase chain reaction were performed to test HMGB1 protein and messenger RNA expression in retinas. We performed TUNEL assays to detect retinal cell apoptosis and electroretinography to detect retinal function. In HRECs treated with high glucose, proliferation, morphology, apoptosis, super-oxide dismutase (SOD), and reactive oxygen species production were detected. Western blot was applied to determine the expressions of HMGB1 and its related protein and apoptosis protein.
Results: Intravitreal injection of HMGB1 siRNA reduced protein and messenger RNA expression of HMGB1 (both P<0.05). Intravitreal injection of HMGB1 siRNA reduced apoptosis of retinal cells (P<0.05), protected morphological changes in the retina, and improved the function of the retina (P<0.05). In HRECs treated with high glucose, HMGB1 siRNA pretreatment increased cell viability, reduced cell apoptosis, and reduced oxidative damage to cells (all P<0.05). Western blot detection found that HMGB1 siRNA pretreatment can inhibit the expression of cleaved caspase 3 and improve the expression of BCL2 (P<0.05). HMGB1 and NFκB expression increased in a time-dependent manner in the high-glucose environment and IKKβ and NFκB protein expression decreased significantly after HMGB1 silencing.
Conclusion: As a therapeutic target, HMGB1 siRNA can reduce retinal cell damage induced by high glucose in vitro and in vivo and delay DR progress through the HMGB1-IKKβ-NFκB signaling pathway.
Keywords: diabetic retinopathy; high-mobility group box 1; human retinal endothelial cells; inhibitor of nuclear factor κB; nuclear factor κB; small interfering RNA.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



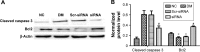


Similar articles
-
Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers.J Physiol Biochem. 2015 Sep;71(3):359-72. doi: 10.1007/s13105-015-0416-x. Epub 2015 Jun 4. J Physiol Biochem. 2015. PMID: 26040511
-
High-Mobility Group Box-1 Modulates the Expression of Inflammatory and Angiogenic Signaling Pathways in Diabetic Retina.Curr Eye Res. 2015;40(11):1141-52. doi: 10.3109/02713683.2014.982829. Epub 2014 Dec 11. Curr Eye Res. 2015. PMID: 25495026
-
Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats.Nutrients. 2016 Mar 3;8(3):140. doi: 10.3390/nu8030140. Nutrients. 2016. PMID: 26950148 Free PMC article.
-
SGLT2 Inhibitor-Induced Low-Grade Ketonemia Ameliorates Retinal Hypoxia in Diabetic Retinopathy-A Novel Hypothesis.J Clin Endocrinol Metab. 2021 Apr 23;106(5):1235-1244. doi: 10.1210/clinem/dgab050. J Clin Endocrinol Metab. 2021. PMID: 33512450 Review.
-
Nutraceuticals for the Treatment of Diabetic Retinopathy.Nutrients. 2019 Apr 2;11(4):771. doi: 10.3390/nu11040771. Nutrients. 2019. PMID: 30987058 Free PMC article. Review.
Cited by
-
Glycyrrhizin Protects the Diabetic Retina against Permeability, Neuronal, and Vascular Damage through Anti-Inflammatory Mechanisms.J Clin Med. 2019 Jul 2;8(7):957. doi: 10.3390/jcm8070957. J Clin Med. 2019. PMID: 31269685 Free PMC article.
-
Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach.Int J Mol Sci. 2020 Mar 28;21(7):2351. doi: 10.3390/ijms21072351. Int J Mol Sci. 2020. PMID: 32231131 Free PMC article. Review.
-
Protective effects of nattokinase against microvasculopathy and neuroinflammation in diabetic retinopathy.J Diabetes. 2023 Oct;15(10):866-880. doi: 10.1111/1753-0407.13439. Epub 2023 Jul 4. J Diabetes. 2023. PMID: 37403338 Free PMC article.
-
Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression.Neural Regen Res. 2021 Jul;16(7):1344-1350. doi: 10.4103/1673-5374.301027. Neural Regen Res. 2021. PMID: 33318415 Free PMC article.
-
High Mobility Group Box-1 and Diabetes Mellitus Complications: State of the Art and Future Perspectives.Int J Mol Sci. 2019 Dec 11;20(24):6258. doi: 10.3390/ijms20246258. Int J Mol Sci. 2019. PMID: 31835864 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

