Fixed-dose combination orally disintegrating tablets to treat cardiovascular disease: formulation, in vitro characterization and physiologically based pharmacokinetic modeling to assess bioavailability
- PMID: 28352156
- PMCID: PMC5358997
- DOI: 10.2147/DDDT.S126035
Fixed-dose combination orally disintegrating tablets to treat cardiovascular disease: formulation, in vitro characterization and physiologically based pharmacokinetic modeling to assess bioavailability
Abstract
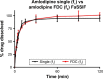
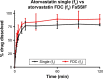
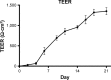
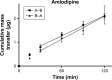
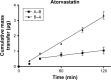
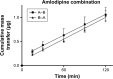
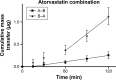
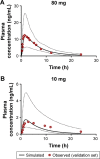
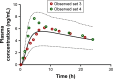
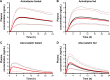
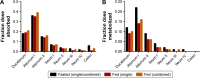
Cardiovascular disease (CVD) is the leading cause of death among men and women worldwide. In CVD, hypertension and dyslipidemia commonly coexist and are managed through coadministration of amlodipine and atorvastatin, respectively. The case for fixed-dose combination (FDC) oral dosage forms and orally disintegrating tablet (ODT) technology to enhance outcomes and compliance is strong. This work follows the development and characterization of single and FDC ODTs containing amlodipine and atorvastatin, followed by bioequivalence comparison between these single and FDC formulations, using in vitro dissolution and Caco-2 apparent permeability (Papp) and in silico physiologically based pharmacokinetic modeling approaches. ODTs containing amlodipine (5 mg) and atorvastatin (10 mg) either alone or in combination rapidly disintegrated (<30 s) while displaying a radial crushing strength in excess of 100 N and friability ≤1%. In vitro dissolution test was performed in fasted and fed-state simulated intestinal fluid (FeSSIF) and analyzed using high-performance liquid chromatography. Dissolution profiles for single and FDC ODTs were compared using US FDA recommended difference (f1) and similarity (f2) factor testing for bioequivalence. In all cases, there was no difference in active pharmaceutical ingredient dissolution between single or FDC ODTs, with the exception of amlodipine in FeSSIF. Pharmacokinetic clinical trial simulations were conducted using Simcyp (Version 14), incorporating Papp and dissolution data. Simulated clinical trials in healthy volunteers showed no difference in bioavailability based on pharmacokinetic parameters between single and combination doses with either active pharmaceutical ingredient. An increase in Cmax and AUC for atorvastatin in fed subjects was attributed to extended transit along the gut lumen and reduced atorvastatin metabolism due to lower CYP3A4 expression at more distal small intestine absorption sites. The results demonstrated bioequivalence of an FDC ODT for amlodipine and atorvastatin, while highlighting several limitations of f1 and f2 bioequivalence testing and strengths of mechanistic pharmacokinetic modeling for oral drug absorption.
Keywords: bioavailability; bioequivalence; cardiovascular disease; fixed-dose combination; orally disintegrating tablet; physiologically based pharmacokinetic modeling.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. - PubMed
-
- Ivanovic B, Tadic M. Fixed combination of amlodipine/atorvastatin from mechanisms to trials. J Cardiovasc Pharmacol Ther. 2013;18(6):544–549. - PubMed
-
- Curran MP. Amlodipine/atorvastatin: a review of its use in the treatment of hypertension and dyslipidaemia and the prevention of cardiovascular disease. Drugs. 2010;70(2):191–213. - PubMed
-
- Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13(1 Pt 2):3s–10s. - PubMed
-
- Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10(12):926–932. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

