High-Fat Feeding Does Not Disrupt Daily Rhythms in Female Mice because of Protection by Ovarian Hormones
- PMID: 28352249
- PMCID: PMC5348546
- DOI: 10.3389/fendo.2017.00044
High-Fat Feeding Does Not Disrupt Daily Rhythms in Female Mice because of Protection by Ovarian Hormones
Abstract
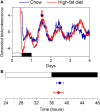
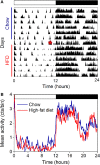
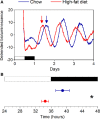
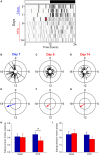
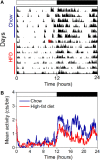
Obesity in women is increased by the loss of circulating estrogen after menopause. Shift work, which disrupts circadian rhythms, also increases the risk for obesity. It is not known whether ovarian hormones interact with the circadian system to protect females from obesity. During high-fat feeding, male C57BL/6J mice develop profound obesity and disruption of daily rhythms. Since C57BL/6J female mice did not develop diet-induced obesity (during 8 weeks of high-fat feeding), we first determined if daily rhythms in female mice were resistant to disruption from high-fat diet. We fed female PERIOD2:LUCIFERASE mice 45% high-fat diet for 1 week and measured daily rhythms. Female mice retained robust rhythms of eating behavior and locomotor activity during high-fat feeding that were similar to chow-fed females. In addition, the phase of the liver molecular timekeeping (PER2:LUC) rhythm was not altered by high-fat feeding in females. To determine if ovarian hormones protected daily rhythms in female mice from high-fat feeding, we analyzed rhythms in ovariectomized mice. During high-fat feeding, the amplitudes of the eating behavior and locomotor activity rhythms were reduced in ovariectomized females. Liver PER2:LUC rhythms were also advanced by ~4 h by high-fat feeding, but not chow, in ovariectomized females. Together these data show circulating ovarian hormones protect the integrity of daily rhythms in female mice during high-fat feeding.
Keywords: C57BL/6J; bioluminescence; circadian; eating rhythm; female; high-fat diet; liver; obesity.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

