Role of microRNA-130a in myocardial hypoxia/reoxygenation injury
- PMID: 28352363
- PMCID: PMC5348690
- DOI: 10.3892/etm.2016.3984
Role of microRNA-130a in myocardial hypoxia/reoxygenation injury
Abstract
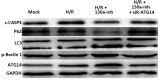
The aim of this study was to investigate the role of microRNA (miR)-130a in the pathogenesis of myocardial hypoxia/reoxygenation (H/R) injury. Primary rat cardiomyocytes were cultured and subjected to H/R treatment. Reverse transcription-quantitative polymerase chain reaction was performed to detect the levels of miR-130a, western blot analysis was used to determine the expression of various proteins, and CCK-8 assay was performed to determine cell viability. In addition, flow cytometry was used to assess apoptosis. The cell viability was significantly decreased and the apoptosis rate was significantly increased in H/R-treated primary cardiomyocytes, and the expression level of miR-130a was also elevated in these model cells. Transfection with miR-130a inhibitor significantly elevated the cell viability and reduced the apoptosis rate in H/R-treated cardiomyocytes. Bioinformatics analysis indicated that autophagy-related gene 14 (ATG14) is the target for miR-130a, which was confirmed by dual-luciferase reporter assay and western blot analysis. When the H/R model cells were co-transfected with miR-130a inhibitor and small interfering RNA against ATG14, the cell viability was significantly reduced and the apoptosis rate was significantly elevated, compared with that of cells transfected with miR-130a inhibitor alone. miR-130a inhibitor transfection significantly elevated the levels of ATG14 and phosphorylated (p-)Beclin 1, increased the LC3II/LC3I ratio, and decreased the expression levels of P62 and cleaved caspase-3, while the co-transfection of miR-130a inhibitor and siR-ATG14 attenuated these effects in H/R-induced primary cardiomyocytes. These results indicate that miR-130a is involved in H/R-induced injuries in primary cardiomyocytes, and that the inhibition of miR-130a increases the levels of ATG14 and p-Beclin 1, thereby increasing autophagy and inhibiting apoptosis in these cells.
Keywords: apoptosis; autophagy; autophagy-related gene 14; hypoxia/reoxygenation; miR–130a; microRNA.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
