Serum Gp96 is a chaperone of complement-C3 during graft-versus-host disease
- PMID: 28352659
- PMCID: PMC5358489
- DOI: 10.1172/jci.insight.90531
Serum Gp96 is a chaperone of complement-C3 during graft-versus-host disease
Abstract
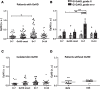
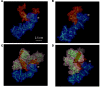
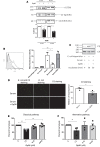
Better identification of severe acute graft-versus-host disease (GvHD) may improve the outcome of this life-threatening complication of allogeneic hematopoietic stem cell transplantation. GvHD induces tissue damage and the release of damage-associated molecular pattern (DAMP) molecules. Here, we analyzed GvHD patients (n = 39) to show that serum heat shock protein glycoprotein 96 (Gp96) could be such a DAMP molecule. We demonstrate that serum Gp96 increases in gastrointestinal GvHD patients and its level correlates with disease severity. An increase in Gp96 serum level was also observed in a mouse model of acute GvHD. This model was used to identify complement C3 as a main partner of Gp96 in the serum. Our biolayer interferometry, yeast two-hybrid and in silico modeling data allowed us to determine that Gp96 binds to a complement C3 fragment encompassing amino acids 749-954, a functional complement C3 hot spot important for binding of different regulators. Accordingly, in vitro experiments with purified proteins demonstrate that Gp96 downregulates several complement C3 functions. Finally, experimental induction of GvHD in complement C3-deficient mice confirms the link between Gp96 and complement C3 in the serum and with the severity of the disease.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

