Impact of Guidance on the Prescription Patterns of G-CSFs for the Prevention of Febrile Neutropenia Following Anticancer Chemotherapy: A Population-Based Utilization Study in the Lazio Region
- PMID: 28353170
- PMCID: PMC5380704
- DOI: 10.1007/s40259-017-0214-9
Impact of Guidance on the Prescription Patterns of G-CSFs for the Prevention of Febrile Neutropenia Following Anticancer Chemotherapy: A Population-Based Utilization Study in the Lazio Region
Abstract
Background: Current guidelines recommend prophylaxis with granulocyte colony-stimulating factors (G-CSFs) for patients with cancer who are at greater risk of febrile neutropenia (FN) while receiving chemotherapy. G-CSF biosimilars are available and represent a savings opportunity; however, their uptake has thus far been low.
Objective: Our objective was to evaluate prescribing patterns for G-CSFs in the prevention of chemotherapy-related FN and to evaluate the impact of regional guidance on G-CSF prescription.
Methods: We conducted an observational drug-utilization study in the Lazio region of Italy using the Electronic Therapeutic Plan Registry, which collects information on G-CSF prescriptions reimbursed by the regional health service. This registry includes information on demographics, tumour, indication for G-CSF use and previous G-CSF exposure. All therapeutic plans (TPs) registered from 1 July 2015 to 30 June 2016 were selected. A pharmaceutical policy intervention was implemented in November 2015. We evaluated temporal trends regarding G-CSF substances and compared the frequency of TPs for each G-CSF substance during the pre- and post-intervention periods.
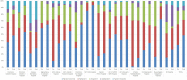
Results: A total of 7082 TPs were eligible for the analysis, corresponding to 6592 patients. The frequency of TPs prescribed after the intervention indicated a significant increase in the use of a filgrastim biosimilar (% difference: 14.4; p < 0.001) and significant decreases in the use of lenograstim (% difference: -6.0; p < 0.001) and pegfilgrastim (% difference: -7.8; p < 0.001). The temporal trends analysis showed an increase in TPs using a filgrastim biosimilar (from 34.4% in July 2015 to 49.8% in June 2016; p < 0.0001) and a decrease in TPs using lenograstim and pegfilgrastim.
Conclusions: This study shows it is possible to change attitudes towards the prescription of less expensive G-CSFs in the FN setting when the prescriber's decision-making processes are supported by evidence that includes both regulatory and clinical information and the analysis of clinical practice data.
Conflict of interest statement
Conflicts of Interest
Francesco Trotta, Flavia Mayer, Alessandra Mecozzi, Laura Amato and Antonio Addis have no conflicts of interest.
Ethical Approval
The study was based on routinely collected data that were retrospectively analysed; therefore, ethical approval was not required. Only anonymised data were used. Informed consent is not required for this type of study.
Funding
Only public employees of the regional health authorities were involved in conceiving, planning, and conducting the study; no additional funding was received.
Author Contributions
All authors made substantial contributions to the conception or design of the work and/or to the acquisition, analysis, or interpretation of the data. All authors were involved in developing and critically revising the content of the manuscript, and all provided final approval of the version submitted for publication. None of the results presented in the current manuscript have been published elsewhere.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

