Consistency of Effect with a Low-Dose, Estradiol Vaginal Capsule (TX-004HR): Evaluating Improvement in Vaginal Physiology and Moderate-to-Severe Dyspareunia in Subgroups of Postmenopausal Women
- PMID: 28355090
- PMCID: PMC5512333
- DOI: 10.1089/jwh.2016.6187
Consistency of Effect with a Low-Dose, Estradiol Vaginal Capsule (TX-004HR): Evaluating Improvement in Vaginal Physiology and Moderate-to-Severe Dyspareunia in Subgroups of Postmenopausal Women
Abstract
Background: The 12-week, randomized, double-blind, placebo-controlled, multicenter, phase 3 REJOICE trial demonstrated that TX-004HR, an investigational, applicator-free, low-dose vaginal softgel capsule containing solubilized 17β-estradiol, effectively and rapidly treats symptoms of vulvar and vaginal atrophy (VVA) with negligible to very low systemic absorption. The aim of this analysis was to assess whether the efficacy of TX-004HR varies with age, body mass index (BMI), uterine status, pregnancy status, and vaginal delivery.
Methods: The REJOICE trial evaluated the efficacy of 4-, 10-, and 25-μg doses of TX-004HR in postmenopausal women (40-75 years) with VVA and a self-identified most bothersome symptom of moderate-to-severe dyspareunia. Prespecified subgroup analyses of the four co-primary endpoints (percentages of superficial cells and parabasal cells, vaginal pH, and severity of dyspareunia) were analyzed with respect to age, BMI, uterine status, pregnancy status, and vaginal births. Each dose was compared with placebo for change from baseline to week 2 through week 12, respectively.
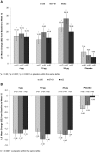
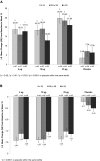
Results: TX-004HR significantly improved superficial cells, parabasal cells, and vaginal pH from baseline to weeks 2 and 12 in most subgroups. All TX-004HR doses numerically reduced the severity of dyspareunia by 2 weeks and maintained efficacy over 12 weeks, with many of the subgroups having statistically significant improvement relative to placebo.
Conclusions: TX-004HR was efficacious for treating symptomatic VVA, and it demonstrated a consistency of effect when women's age, BMI, uterine status, pregnancy status, and vaginal births were evaluated. Clinical Trial Identifier: NCT02253173.
Keywords: 17-β estradiol; age; body mass index; dyspareunia; vaginal atrophy.
Conflict of interest statement
Dr. Constantine consults to multiple pharmaceutical companies, including, but not limited to, TherapeuticsMD and has stock options with TherapeuticsMD. Dr. Bouchard has received research grants from TherapeuticsMD, Merck Canada, GlaxoSmithKline, Bayer, and Endoceutics and an educational grant from Merck Canada. Dr. Pickar has received consultant fees from Wyeth/Pfizer, Radius Health Inc., Shionogi Inc., and TherapeuticsMD; and has stock options with TherapeuticsMD. Dr. Archer (within the past 3 years) has received research support from Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Bayer Healthcare, Endoceutics, Glenmark, Merck (previously Schering Plough, Organon), Radius Health Inc., Shionogi Inc., and TherapeuticsMD; and has served as a consultant to Abbvie (previously Abbott Laboratories), Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis (previously CHEMO), InnovaGyn, Merck (previously Schering Plough, Organon), Pfizer, Radius Health Inc., Sermonix Pharmaceuticals, Shionogi Inc., Teva Women's Healthcare, and TherapeuticsMD. Dr. Bernick is a board member and an employee of TherapeuticsMD with stock/stock options. Dr. Graham and Dr. Mirkin are employees of TherapeuticsMD with stock/stock options.
Figures


References
-
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014;21:1063–1068 - PubMed
-
- Lev-Sagie A. Vulvar and vaginal atrophy: Physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol 2015;58:476–491 - PubMed
-
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013;20:888–902 - PubMed
-
- Constantine G, Simon JA, Pickar JH, et al. The REJOICE trial: A phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol softgel capsule for symptomatic vulvar and vaginal atrophy (VVA). Menopause. 2016. [Epub ahead of print]; DOI: 10.1097/GME.0000000000000786 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

