Immunization with a streptococcal multiple-epitope recombinant protein protects mice against invasive group A streptococcal infection
- PMID: 28355251
- PMCID: PMC5371370
- DOI: 10.1371/journal.pone.0174464
Immunization with a streptococcal multiple-epitope recombinant protein protects mice against invasive group A streptococcal infection
Abstract
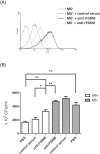
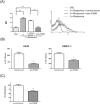
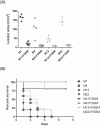
Streptococcus pyogenes (group A Streptococcus; GAS) causes clinical diseases, including pharyngitis, scarlet fever, impetigo, necrotizing fasciitis and streptococcal toxic shock syndrome. A number of group A streptococcus vaccine candidates have been developed, but only one 26-valent recombinant M protein vaccine has entered clinical trials. Differing from the design of a 26-valent recombinant M protein vaccine, we provide here a vaccination using the polyvalence epitope recombinant FSBM protein (rFSBM), which contains four different epitopes, including the fibronectin-binding repeats domain of streptococcal fibronectin binding protein Sfb1, the C-terminal immunogenic segment of streptolysin S, the C3-binding motif of streptococcal pyrogenic exotoxin B, and the C-terminal conserved segment of M protein. Vaccination with the rFSBM protein successfully prevented mortality and skin lesions caused by several emm strains of GAS infection. Anti-FSBM antibodies collected from the rFSBM-immunized mice were able to opsonize at least six emm strains and can neutralize the hemolytic activity of streptolysin S. Furthermore, the internalization of GAS into nonphagocytic cells is also reduced by anti-FSBM serum. These findings suggest that rFSBM can be applied as a vaccine candidate to prevent different emm strains of GAS infection.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

