To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post kala-azar dermal leishmaniasis (PKDL)
- PMID: 28355259
- PMCID: PMC5371363
- DOI: 10.1371/journal.pone.0174497
To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post kala-azar dermal leishmaniasis (PKDL)
Abstract
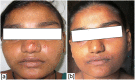
Background: Post kala-azar dermal leishmaniasis (PKDL) is a skin disorder that usually occurs among patients with a past history of visceral leishmaniasis (VL). Cases are also reported without a history of VL. There is no satisfactory treatment regimen available at present. We aimed to compare the efficacy and safety of amphotericin B in two different doses (0.5mg/kg vs 1mg/kg) in a prospective randomized trial in 50 PKDL patients.
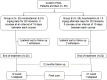
Methods: In this open label study 50 patients with PKDL, aged between 5-60 years were randomized in two groups. Group A received amphotericin B in the dose of 0.5 mg/kg in 5% dextrose, daily for 20 infusions for 3 courses at an interval of 15 days between each course and Group B received amphotericin B in the dose of 1mg/kg in 5% dextrose on alternate days, 20 infusions for 3 courses an interval of 15 days between each course and followed up for one year.
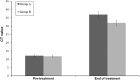
Results: A total of 50 patients were enrolled, 25 in each of group A and group B. Two patients lost to follow up and three patients withdrew consent due to adverse events. The initial cure rate was 92% in group A and 88% in group B by intention to treat analysis and final cure rate by per protocol analysis was 95.65% and 95.45% in group A and group B respectively. Two patients each from either group relapsed. Nephrotoxicity was the most common adverse event occurring in both the groups.
Conclusion: The lower dose appears to have fewer adverse events however, nephrotoxicity remains a problem in both regimens. The 0.5mg/kg regimen may be considered instead of the higher dosage however safer treatments remain critical for PKDL treatment.
Conflict of interest statement
Figures

References
-
- El Hassan AM, Khalil EA. Post-kala-azar dermal leishmaniasis: does it play a role in the transmission of Leishmania donovani in the Sudan? Trop Med Int Health 2001;6: 743–744. - PubMed
-
- Post-kala-azar dermal leishmaniasis: a manual for case management and control: report of a WHO consultative meeting, Kolkata, India, 2–3 July 2012.
-
- Saha S, Mondal S, Ravindran R, Bhowmick S, Modak D, Mallick S, et al. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol. 207;179: 5592–5603. - PubMed
-
- Das VNR, Pandey K, Verma N, Lal CS, Bimal S, Topno RK, et al. Development of Post–Kala-Azar Dermal Leishmaniasis (PKDL) in Miltefosine-Treated Visceral Leishmaniasis. Am J Trop Med Hyg. 2009;80: 336–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

