Structural basis for non-genuine phenolic acceptor substrate specificity of Streptomyces roseochromogenes prenyltransferase CloQ from the ABBA/PT-barrel superfamily
- PMID: 28355308
- PMCID: PMC5371340
- DOI: 10.1371/journal.pone.0174665
Structural basis for non-genuine phenolic acceptor substrate specificity of Streptomyces roseochromogenes prenyltransferase CloQ from the ABBA/PT-barrel superfamily
Abstract
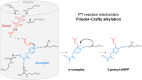
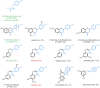
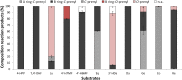
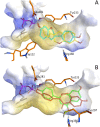
Acceptor substrate specificity of Streptomyces roseochromogenes prenyltransferase SrCloQ was investigated using different non-genuine phenolic compounds. RP-UHPLC-UV-MSn was used for the tentative annotation and quantification of the prenylated products. Flavonoids, isoflavonoids and stilbenoids with different types of substitution were prenylated by SrCloQ, although with less efficiency than the genuine substrate 4-hydroxyphenylpyruvate. The isoflavan equol, followed by the flavone 7,4'-dihydroxyflavone, were the best non-genuine acceptor substrates. B-ring C-prenylation was in general preferred over A-ring C-prenylation (ratio 5:1). Docking studies of non-genuine acceptor substrates with the B-ring oriented towards the donor substrate dimethylallyl pyrophosphate, showed that the carbonyl group of the C-ring was able to make stabilizing interactions with the residue Arg160, which might determine the preference observed for B-ring prenylation. No reaction products were formed when the acceptor substrate had no phenolic hydroxyl groups. This preference can be explained by the essential hydrogen bond needed between a phenolic hydroxyl group and the residue Glu281. Acceptor substrates with an additional hydroxyl group at the C3' position (B-ring), were mainly O3'-prenylated (> 80% of the reaction products). This can be explained by the proximity of the C3' hydroxyl group to the donor substrate at the catalytic site. Flavones were preferred over isoflavones by SrCloQ. Docking studies suggested that the orientation of the B-ring and of the phenolic hydroxyl group at position C7 (A-ring) of flavones towards the residue Tyr233 plays an important role in this observed preference. Finally, the insights obtained on acceptor substrate specificity and regioselectivity for SrCloQ were extended to other prenyltransferases from the CloQ/NhpB family.
Conflict of interest statement
Figures


References
-
- Liang PH, Ko TP, Wang AHJ. Structure, mechanism and function of prenyltransferases. Eur J Biochem. 2002;269(14):3339–54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

