Opposing Intermolecular Tuning of Ca2+ Affinity for Calmodulin by Neurogranin and CaMKII Peptides
- PMID: 28355539
- PMCID: PMC5374985
- DOI: 10.1016/j.bpj.2017.01.020
Opposing Intermolecular Tuning of Ca2+ Affinity for Calmodulin by Neurogranin and CaMKII Peptides
Abstract
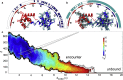
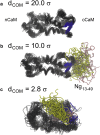
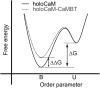
We investigated the impact of bound calmodulin (CaM)-target compound structure on the affinity of calcium (Ca2+) by integrating coarse-grained models and all-atomistic simulations with nonequilibrium physics. We focused on binding between CaM and two specific targets, Ca2+/CaM-dependent protein kinase II (CaMKII) and neurogranin (Ng), as they both regulate CaM-dependent Ca2+ signaling pathways in neurons. It was shown experimentally that Ca2+/CaM (holoCaM) binds to the CaMKII peptide with overwhelmingly higher affinity than Ca2+-free CaM (apoCaM); the binding of CaMKII peptide to CaM in return increases the Ca2+ affinity for CaM. However, this reciprocal relation was not observed in the Ng peptide (Ng13-49), which binds to apoCaM or holoCaM with binding affinities of the same order of magnitude. Unlike the holoCaM-CaMKII peptide, whose structure can be determined by crystallography, the structural description of the apoCaM-Ng13-49 is unknown due to low binding affinity, therefore we computationally generated an ensemble of apoCaM-Ng13-49 structures by matching the changes in the chemical shifts of CaM upon Ng13-49 binding from nuclear magnetic resonance experiments. Next, we computed the changes in Ca2+ affinity for CaM with and without binding targets in atomistic models using Jarzynski's equality. We discovered the molecular underpinnings of lowered affinity of Ca2+ for CaM in the presence of Ng13-49 by showing that the N-terminal acidic region of Ng peptide pries open the β-sheet structure between the Ca2+ binding loops particularly at C-domain of CaM, enabling Ca2+ release. In contrast, CaMKII peptide increases Ca2+ affinity for the C-domain of CaM by stabilizing the two Ca2+ binding loops. We speculate that the distinctive structural difference in the bound complexes of apoCaM-Ng13-49 and holoCaM-CaMKII delineates the importance of CaM's progressive mechanism of target binding on its Ca2+ binding affinities.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007;405:199–221. - PubMed
-
- Yamniuk A.P., Vogel H.J. Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol. 2004;27:33–57. - PubMed
-
- Xia Z., Storm D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005;6:267–276. - PubMed
-
- Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II--discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol. Pharm. Bull. 2005;28:1342–1354. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

