Altered Synapse Stability in the Early Stages of Tauopathy
- PMID: 28355559
- PMCID: PMC5382238
- DOI: 10.1016/j.celrep.2017.03.013
Altered Synapse Stability in the Early Stages of Tauopathy
Abstract
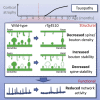
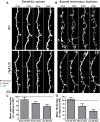
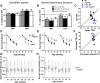
Synapse loss is a key feature of dementia, but it is unclear whether synaptic dysfunction precedes degenerative phases of the disease. Here, we show that even before any decrease in synapse density, there is abnormal turnover of cortical axonal boutons and dendritic spines in a mouse model of tauopathy-associated dementia. Strikingly, tauopathy drives a mismatch in synapse turnover; postsynaptic spines turn over more rapidly, whereas presynaptic boutons are stabilized. This imbalance between pre- and post-synaptic stability coincides with reduced synaptically driven neuronal activity in pre-degenerative stages of the disease.
Keywords: 2-photon microscopy; axon; bouton; cortex; dementia; dendritic spine; neurodegeneration.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Amyotrophic lateral sclerosis mutant TDP-43 may cause synaptic dysfunction through altered dendritic spine function.Dis Model Mech. 2019 May 17;12(5):dmm038109. doi: 10.1242/dmm.038109. Dis Model Mech. 2019. PMID: 31036551 Free PMC article.
-
Sex Impact on Tau-Aggregation and Postsynaptic Protein Levels in the P301L Mouse Model of Tauopathy.J Alzheimers Dis. 2017;56(4):1279-1292. doi: 10.3233/JAD-161087. J Alzheimers Dis. 2017. PMID: 28157099
-
Sleep Deprivation by Exposure to Novel Objects Increases Synapse Density and Axon-Spine Interface in the Hippocampal CA1 Region of Adolescent Mice.J Neurosci. 2019 Aug 21;39(34):6613-6625. doi: 10.1523/JNEUROSCI.0380-19.2019. Epub 2019 Jul 1. J Neurosci. 2019. PMID: 31263066 Free PMC article.
-
Dendritic and synaptic protection: is it enough to save the retinal ganglion cell body and axon?J Neuroophthalmol. 2008 Jun;28(2):144-54. doi: 10.1097/WNO.0b013e318177edf0. J Neuroophthalmol. 2008. PMID: 18562848 Review.
-
The Role of Tau in the Post-synapse.Adv Exp Med Biol. 2019;1184:113-121. doi: 10.1007/978-981-32-9358-8_10. Adv Exp Med Biol. 2019. PMID: 32096033 Review.
Cited by
-
Low Intensity Electromagnetic Fields Act via Voltage-Gated Calcium Channel (VGCC) Activation to Cause Very Early Onset Alzheimer's Disease: 18 Distinct Types of Evidence.Curr Alzheimer Res. 2022;19(2):119-132. doi: 10.2174/1567205019666220202114510. Curr Alzheimer Res. 2022. PMID: 35114921 Free PMC article.
-
Neuroligin-1 in brain and CSF of neurodegenerative disorders: investigation for synaptic biomarkers.Acta Neuropathol Commun. 2021 Feb 1;9(1):19. doi: 10.1186/s40478-021-01119-4. Acta Neuropathol Commun. 2021. PMID: 33522967 Free PMC article.
-
Fluid Biomarkers for Synaptic Dysfunction and Loss.Biomark Insights. 2020 Aug 21;15:1177271920950319. doi: 10.1177/1177271920950319. eCollection 2020. Biomark Insights. 2020. PMID: 32913390 Free PMC article. Review.
-
Model-Based Inference of Synaptic Transmission.Front Synaptic Neurosci. 2019 Aug 20;11:21. doi: 10.3389/fnsyn.2019.00021. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31481887 Free PMC article. Review.
-
Synaptic aging disrupts synaptic morphology and function in cerebellar Purkinje cells.Neural Regen Res. 2018 Jun;13(6):1019-1025. doi: 10.4103/1673-5374.233445. Neural Regen Res. 2018. PMID: 29926829 Free PMC article.
References
-
- Crimins J.L., Rocher A.B., Peters A., Shultz P., Lewis J., Luebke J.I. Homeostatic responses by surviving cortical pyramidal cells in neurodegenerative tauopathy. Acta Neuropathol. 2011;122:551–564. - PubMed
-
- De Paola V., Holtmaat A., Knott G., Song S., Wilbrecht L., Caroni P., Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

