Identification of Interleukin-1 by Functional Screening as a Key Mediator of Cellular Expansion and Disease Progression in Acute Myeloid Leukemia
- PMID: 28355571
- PMCID: PMC5437102
- DOI: 10.1016/j.celrep.2017.03.018
Identification of Interleukin-1 by Functional Screening as a Key Mediator of Cellular Expansion and Disease Progression in Acute Myeloid Leukemia
Abstract
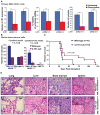
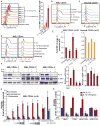
Secreted proteins in the bone marrow microenvironment play critical roles in acute myeloid leukemia (AML). Through an ex vivo functional screen of 94 cytokines, we identified that the pro-inflammatory cytokine interleukin-1 (IL-1) elicited profound expansion of myeloid progenitors in ∼67% of AML patients while suppressing the growth of normal progenitors. Levels of IL-1β and IL-1 receptors were increased in AML patients, and silencing of the IL-1 receptor led to significant suppression of clonogenicity and in vivo disease progression. IL-1 promoted AML cell growth by enhancing p38MAPK phosphorylation and promoting secretion of various other growth factors and inflammatory cytokines. Treatment with p38MAPK inhibitors reversed these effects and recovered normal CD34+ cells from IL-1-mediated growth suppression. These results highlight the importance of ex vivo functional screening to identify common and actionable extrinsic pathways in genetically heterogeneous malignancies and provide impetus for clinical development of IL-1/IL1R1/p38MAPK pathway-targeted therapies in AML.
Keywords: AML; IL1R1; bone marrow microenvironment; functional screening; interleukin-1; p38MAPK.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Agarwal A, MacKenzie RJ, Eide CA, Davare MA, Watanabe-Smith K, Tognon CE, Mongoue-Tchokote S, Park B, Braziel RM, Tyner JW, Druker BJ. Functional RNAi screen targeting cytokine and growth factor receptors reveals oncorequisite role for interleukin-2 gamma receptor in JAK3-mutation-positive leukemia. Oncogene 2014b - PMC - PubMed
-
- Agerstam H, Hansen N, von Palffy S, Sanden C, Reckzeh K, Karlsson C, Lilljebjorn H, Landberg N, Askmyr M, Hogberg C, et al. IL1RAP antibodies block IL-1-induced expansion of candidate CML stem cells and mediate cell killing in xenograft models. Blood. 2016;128:2683–2693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

