Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models
- PMID: 28356148
- PMCID: PMC5372261
- DOI: 10.1186/s12916-017-0827-3
Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models
Abstract
Background: Unexpected clinical deterioration before 34 weeks gestation is an undesired course in early-onset pre-eclampsia. To safely prolong preterm gestation, accurate and timely prediction of complications is required.
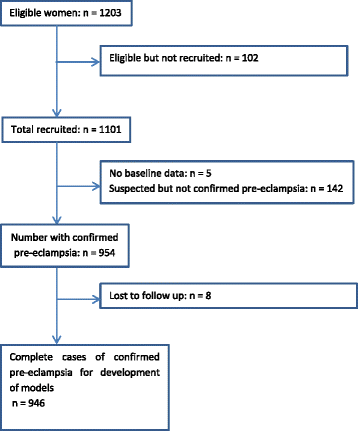
Method: Women with confirmed early onset pre-eclampsia were recruited from 53 maternity units in the UK to a large prospective cohort study (PREP-946) for development of prognostic models for the overall risk of experiencing a complication using logistic regression (PREP-L), and for predicting the time to adverse maternal outcome using a survival model (PREP-S). External validation of the models were carried out in a multinational cohort (PIERS-634) and another cohort from the Netherlands (PETRA-216). Main outcome measures were C-statistics to summarise discrimination of the models and calibration plots and calibration slopes.
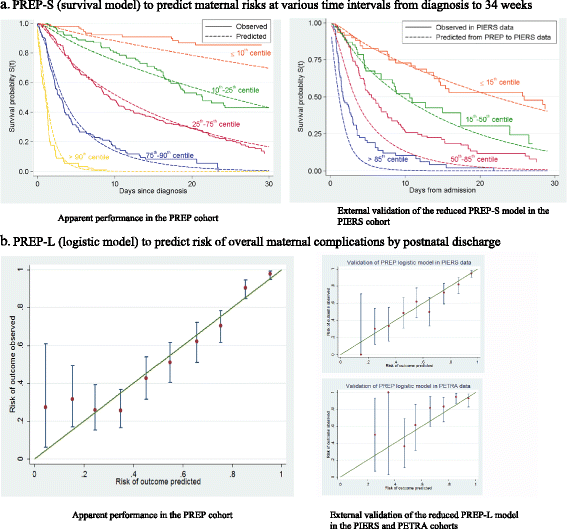
Results: A total of 169 mothers (18%) in the PREP dataset had adverse outcomes by 48 hours, and 633 (67%) by discharge. The C-statistics of the models for predicting complications by 48 hours and by discharge were 0.84 (95% CI, 0.81-0.87; PREP-S) and 0.82 (0.80-0.84; PREP-L), respectively. The PREP-S model included maternal age, gestation, medical history, systolic blood pressure, deep tendon reflexes, urine protein creatinine ratio, platelets, serum alanine amino transaminase, urea, creatinine, oxygen saturation and treatment with antihypertensives or magnesium sulfate. The PREP-L model included the above except deep tendon reflexes, serum alanine amino transaminase and creatinine. On validation in the external PIERS dataset, the reduced PREP-S model showed reasonable calibration (slope 0.80) and discrimination (C-statistic 0.75) for predicting adverse outcome by 48 hours. Reduced PREP-L model showed excellent calibration (slope: 0.93 PIERS, 0.90 PETRA) and discrimination (0.81 PIERS, 0.75 PETRA) for predicting risk by discharge in the two external datasets.
Conclusions: PREP models can be used to obtain predictions of adverse maternal outcome risk, including early preterm delivery, by 48 hours (PREP-S) and by discharge (PREP-L), in women with early onset pre-eclampsia in the context of current care. They have a potential role in triaging high-risk mothers who may need transfer to tertiary units for intensive maternal and neonatal care.
Trial registration: ISRCTN40384046 , retrospectively registered.
Keywords: Complications; Early-onset; Maternal; Pre-eclampsia; Prognostic models.
Figures
References
-
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–8. - PubMed
-
- von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton-Pipkin F, Cote AM, Douglas MJ, Gruslin A, Hutcheon JA, Joseph KS, Kyle PM, Lee T, Loughna P, Menzies JM, Merialdi M, Millman AL, Moore MP, Moutquin JM, Ouellet AB, Smith GN, Walker JJ, Walley KR, Walters BN, Widmer M, Lee SK, Russell JA, Magee LA, PIERS Study Group Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377(9761):219–27. doi: 10.1016/S0140-6736(10)61351-7. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous