Hemoglobin inhibits albumin uptake by proximal tubule cells: implications for sickle cell disease
- PMID: 28356267
- PMCID: PMC5494590
- DOI: 10.1152/ajpcell.00021.2017
Hemoglobin inhibits albumin uptake by proximal tubule cells: implications for sickle cell disease
Abstract
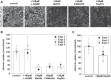
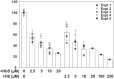
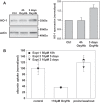
Proximal tubule (PT) dysfunction, including tubular proteinuria, is a significant complication in young sickle cell disease (SCD) that can eventually lead to chronic kidney disease. Hemoglobin (Hb) dimers released from red blood cells upon hemolysis are filtered into the kidney and internalized by megalin/cubilin receptors into PT cells. The PT is especially sensitive to heme toxicity, and tubular dysfunction in SCD is thought to result from prolonged exposure to filtered Hb. Here we show that concentrations of Hb predicted to enter the tubule lumen during hemolytic crisis competitively inhibit the uptake of another megalin/cubilin ligand (albumin) by PT cells. These effects were independent of heme reduction state. The Glu7Val mutant of Hb that causes SCD was equally effective at inhibiting albumin uptake compared with wild-type Hb. Addition of the Hb scavenger haptoglobin (Hpt) restored albumin uptake in the presence of Hb, suggesting that Hpt binding to the Hb αβ dimer-dimer interface interferes with Hb binding to megalin/cubilin. BLAST searches and structural modeling analyses revealed regions of similarity between Hb and albumin that map to this region and may represent sites of Hb interaction with megalin/cubilin. Our studies suggest that impaired endocytosis of megalin/cubilin ligands, rather than heme toxicity, may be the cause of tubular proteinuria in SCD patients. Additionally, loss of these filtered proteins into the urine may contribute to the extra-renal pathogenesis of SCD.
Keywords: megalin; proteinuria; proximal tubule; sickle cell disease; vitamin D.
Copyright © 2017 the American Physiological Society.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

