RGC-32 Promotes Th17 Cell Differentiation and Enhances Experimental Autoimmune Encephalomyelitis
- PMID: 28356385
- PMCID: PMC6197070
- DOI: 10.4049/jimmunol.1602158
RGC-32 Promotes Th17 Cell Differentiation and Enhances Experimental Autoimmune Encephalomyelitis
Abstract
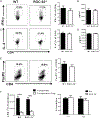
Th17 cells play a critical role in autoimmune diseases, including multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis. Response gene to complement (RGC)-32 is a cell cycle regulator and a downstream target of TGF-β that mediates its profibrotic activity. In this study, we report that RGC-32 is preferentially upregulated during Th17 cell differentiation. RGC-32-/- mice have normal Th1, Th2, and regulatory T cell differentiation but show defective Th17 differentiation in vitro. The impaired Th17 differentiation is associated with defects in IFN regulatory factor 4, B cell-activating transcription factor, retinoic acid-related orphan receptor γt, and SMAD2 activation. In vivo, RGC-32-/- mice display an attenuated experimental autoimmune encephalomyelitis phenotype accompanied by decreased CNS inflammation and reduced frequency of IL-17- and GM-CSF-producing CD4+ T cells. Collectively, our results identify RGC-32 as a novel regulator of Th17 cell differentiation in vitro and in vivo and suggest that RGC-32 is a potential therapeutic target in multiple sclerosis and other Th17-mediated autoimmune diseases.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures


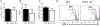

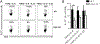

Similar articles
-
T-bet is essential for Th1-mediated, but not Th17-mediated, CNS autoimmune disease.Eur J Immunol. 2013 Nov;43(11):2818-23. doi: 10.1002/eji.201343689. Epub 2013 Aug 21. Eur J Immunol. 2013. PMID: 23878019 Free PMC article.
-
Simvastatin ameliorates experimental autoimmune encephalomyelitis by inhibiting Th1/Th17 response and cellular infiltration.Inflammopharmacology. 2015 Dec;23(6):343-54. doi: 10.1007/s10787-015-0252-1. Epub 2015 Nov 11. Inflammopharmacology. 2015. PMID: 26559850
-
Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis.Clin Exp Immunol. 2012 Oct;170(1):66-76. doi: 10.1111/j.1365-2249.2012.04637.x. Clin Exp Immunol. 2012. PMID: 22943202 Free PMC article.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Th17 Cells in MS and Experimental Autoimmune Encephalomyelitis.Int MS J. 2009 Apr;16(1):12-8. Int MS J. 2009. PMID: 19413921 Review.
Cited by
-
RGC-32 Regulates Generation of Reactive Astrocytes in Experimental Autoimmune Encephalomyelitis.Front Immunol. 2021 Jan 25;11:608294. doi: 10.3389/fimmu.2020.608294. eCollection 2020. Front Immunol. 2021. PMID: 33569054 Free PMC article.
-
RGC-32 and diseases: the first 20 years.Immunol Res. 2019 Jun;67(2-3):267-279. doi: 10.1007/s12026-019-09080-0. Immunol Res. 2019. PMID: 31250246 Review.
-
Regulator of Cell Cycle Protein (RGCC/RGC-32) Protects against Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2022 Feb;66(2):146-157. doi: 10.1165/rcmb.2021-0022OC. Am J Respir Cell Mol Biol. 2022. PMID: 34668840 Free PMC article.
-
Differential neurodegenerative phenotypes are associated with heterogeneous voiding dysfunction in a coronavirus-induced model of multiple sclerosis.Sci Rep. 2019 Jul 26;9(1):10869. doi: 10.1038/s41598-019-47407-x. Sci Rep. 2019. PMID: 31350464 Free PMC article.
-
RGC-32 regulates reactive astrocytosis and extracellular matrix deposition in experimental autoimmune encephalomyelitis.Immunol Res. 2018 Aug;66(4):445-461. doi: 10.1007/s12026-018-9011-x. Immunol Res. 2018. PMID: 30006805 Free PMC article.
References
-
- Badea TC, Niculescu FI, Soane L, Shin ML, and Rus H. 1998. Molecularcloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J. Biol. Chem 273: 26977–26981. - PubMed
-
- Tegla CA, Cudrici CD, Azimzadeh P, Singh AK, Trippe R III, Khan A,Chen H, Andrian-Albescu M, Royal W III, Bever C, et al. 2013. Dual role of response gene to complement-32 in multiple sclerosis. Exp. Mol. Pathol 94: 17–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

