Clustering of integrin α5 at the lateral membrane restores epithelial polarity in invasive colorectal cancer cells
- PMID: 28356422
- PMCID: PMC5426844
- DOI: 10.1091/mbc.E16-12-0852
Clustering of integrin α5 at the lateral membrane restores epithelial polarity in invasive colorectal cancer cells
Abstract
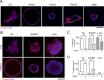
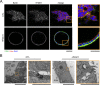
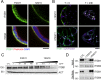
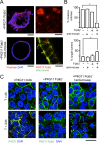
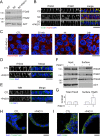
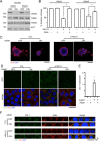
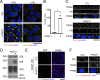
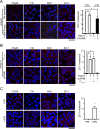
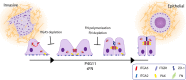
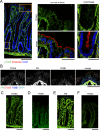
Apicobasolateral polarity is a fundamental property of epithelial cells, and its loss is a hallmark of cancer. Integrin-mediated contact with the extracellular matrix defines the basal surface, setting in motion E-cadherin-mediated cell-cell contact, which establishes apicobasolateral polarity. Role(s) for lateral integrins in this polarization process and the consequences of their disruption are incompletely understood. We show that addition of an integrin β1-activating monoclonal antibody, P4G11, to invasive colorectal cancer cells in three-dimensional type 1 collagen reverts the invasive phenotype and restores apicobasolateral polarity. P4G11 induces clustering of integrin α5β1 at lateral, intercellular surfaces. This leads to deposition and polymerization of fibronectin and recruitment of paxillin to sites of lateral integrin α5β1 clustering and is followed by tight junction formation, as determined by ZO-1 localization. Inducible elimination of integrin α5 abrogates the epithelial-organizing effects of P4G11. In addition, polymerization of fibronectin is required for the effects of P4G11, and addition of polymerized superfibronectin is sufficient to induce tight junction formation and apicobasolateral polarization. In the normal human colon, we show that integrin α5 localizes to the lateral membrane of terminally differentiated colonocytes and that integrin α5 staining may be reduced in colorectal cancer. Thus we propose a novel role for integrin α5β1 in regulating epithelial morphogenesis.
© 2017 Starchenko et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
Alpha5beta1 integrin mediates strong tissue cohesion.J Cell Sci. 2003 Jan 15;116(Pt 2):377-86. doi: 10.1242/jcs.00231. J Cell Sci. 2003. PMID: 12482923
-
IL-1β promotes transendothelial migration of PBMCs by upregulation of the FN/α5β1 signalling pathway in immortalised human brain microvascular endothelial cells.Exp Cell Res. 2018 Dec 15;373(1-2):99-111. doi: 10.1016/j.yexcr.2018.10.002. Epub 2018 Oct 19. Exp Cell Res. 2018. PMID: 30342992
-
α5β1 integrin mediates pulmonary epithelial cyst formation.Dev Dyn. 2017 Jun;246(6):475-484. doi: 10.1002/dvdy.24502. Epub 2017 Apr 29. Dev Dyn. 2017. PMID: 28370666 Free PMC article.
-
Integrins in epithelial cell polarity: using antibodies to analyze adhesive function and morphogenesis.Methods. 2003 Jul;30(3):235-46. doi: 10.1016/s1046-2023(03)00030-6. Methods. 2003. PMID: 12798138 Review.
-
Integrins and epithelial cell polarity.J Cell Sci. 2014 Aug 1;127(Pt 15):3217-25. doi: 10.1242/jcs.146142. Epub 2014 Jul 2. J Cell Sci. 2014. PMID: 24994933 Free PMC article. Review.
Cited by
-
Apical integrins as a switchable target to regulate the epithelial barrier.J Cell Sci. 2024 Dec 15;137(24):jcs263580. doi: 10.1242/jcs.263580. Epub 2024 Dec 20. J Cell Sci. 2024. PMID: 39552289 Free PMC article.
-
BMAL1 Knockdown Leans Epithelial-Mesenchymal Balance toward Epithelial Properties and Decreases the Chemoresistance of Colon Carcinoma Cells.Int J Mol Sci. 2021 May 16;22(10):5247. doi: 10.3390/ijms22105247. Int J Mol Sci. 2021. PMID: 34065633 Free PMC article.
-
Long non-coding RNA ABHD11-AS1 promotes colorectal cancer progression and invasion through targeting the integrin subunit alpha 5/focal adhesion kinase/phosphoinositide 3 kinase/Akt signaling pathway.Aging (Albany NY). 2021 Aug 10;13(16):20179-20191. doi: 10.18632/aging.203342. Epub 2021 Aug 10. Aging (Albany NY). 2021. PMID: 34375304 Free PMC article.
-
Cell surface integrin α5ß1 clustering negatively regulates receptor tyrosine kinase signaling in colorectal cancer cells via glycogen synthase kinase 3.Integr Biol (Camb). 2021 Jun 15;13(6):153-166. doi: 10.1093/intbio/zyab009. Integr Biol (Camb). 2021. PMID: 34037774 Free PMC article.
-
Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids.Biomaterials. 2020 Sep;254:120125. doi: 10.1016/j.biomaterials.2020.120125. Epub 2020 May 25. Biomaterials. 2020. PMID: 32502894 Free PMC article.
References
-
- Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183:363–369. - PubMed
-
- Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14:430–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

