PICs in motoneurons do not scale with the size of the animal: a possible mechanism for faster speed of muscle contraction in smaller species
- PMID: 28356469
- PMCID: PMC5494365
- DOI: 10.1152/jn.00045.2017
PICs in motoneurons do not scale with the size of the animal: a possible mechanism for faster speed of muscle contraction in smaller species
Abstract
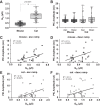
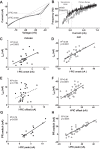
The majority of studies on the electrical properties of neurons are carried out in rodents, and in particular in mice. However, the minute size of this animal compared with humans potentially limits the relevance of the resulting insights. To be able to extrapolate results obtained in a small animal such as a rodent, one needs to have proper knowledge of the rules governing how electrical properties of neurons scale with the size of the animal. Generally speaking, electrical resistances of neurons increase as cell size decreases, and thus maintenance of equal depolarization across cells of different sizes requires the underlying currents to decrease in proportion to the size decrease. Thus it would generally be expected that voltage-sensitive currents are smaller in smaller animals. In this study, we used in vivo preparations to record electrical properties of spinal motoneurons in deeply anesthetized adult mice and cats. We found that PICs do not scale with size, but instead are constant in their amplitudes across these species. This constancy, coupled with the threefold differences in electrical resistances, means that PICs contribute a threefold larger depolarization in the mouse than in the cat. As a consequence, motoneuronal firing rate sharply increases as animal size decreases. These differences in firing rates are likely essential in allowing different species to control muscles with widely different contraction speeds (smaller animals have faster muscle fibers). Thus from our results we have identified a possible new mechanism for how electrical properties are tuned to match mechanical properties within the motor output system.NEW & NOTEWORTHY The small size of the mouse warrants concern over whether the properties of their neurons are a scaled version of those in larger animals or instead have unique features. Comparison of spinal motoneurons in mice to cats showed unique features. Firing rates in the mouse were much higher, in large part due to relatively larger persistent inward currents. These differences likely reflect adaptations for controlling much faster muscle fibers in mouse than cat.
Keywords: adult spinal motoneurons; electrical properties; persistent inward currents; voltage clamp.
Copyright © 2017 the American Physiological Society.
Figures




References
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2038–2045, 1998a. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998b. - PubMed
-
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001. - PubMed
-
- Burke R.. Motor units: anatomy, physiology, and functional organization. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: American Physiological Society, 1981, p. 345–421.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

