SK channel inhibition mediates the initiation and amplitude modulation of synchronized burst firing in the spinal cord
- PMID: 28356481
- PMCID: PMC5494358
- DOI: 10.1152/jn.00929.2016
SK channel inhibition mediates the initiation and amplitude modulation of synchronized burst firing in the spinal cord
Abstract
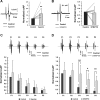
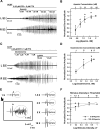
Burst firing in motoneurons represents the basis for generating meaningful movements. Neuromodulators and inhibitory receptor blocker cocktails have been used for years to induce burst firing in vitro; however, the ionic mechanisms in the motoneuron membrane that contribute to burst initiation and amplitude modulation are not fully understood. Small conductance Ca2+-activated potassium (SK) channels regulate excitatory inputs and firing output of motoneurons and interneurons and therefore, are a candidate for mediating bursting behavior. The present study examines the role of SK channels in the generation of synchronized bursting using an in vitro spinal cord preparation from adult mice. Our results show that SK channel inhibition is required for both initiation and amplitude modulation of burst firing. Specifically, administration of the synaptic inhibition blockers strychnine and picrotoxin amplified the spinal circuit excitatory drive but not enough to evoke bursting. However, when SK channels were inhibited using various approaches, the excitatory drive was further amplified, and synchronized bursting was always evoked. Furthermore, graded SK channel inhibition modulated the amplitude of the burst in a dose-dependent manner, which was reversed using SK channel activators. Importantly, modulation of neuronal excitability using multiple approaches failed to mimic the effects of SK modulators, suggesting a specific role for SK channel inhibition in generating bursting. Both NMDA (N-methyl-d-aspartate) and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors were found to drive the synchronized bursts. The blocking of gap junctions did not disturb the burst synchrony. These results demonstrate a novel mechanistic role for SK channels in initiating and modulating burst firing of spinal motoneurons.NEW & NOTEWORTHY This study demonstrates that cholinergic inhibition or direct blockade of small conductance Ca2+-activated potassium (SK) channels facilitates burst firing in spinal motoneurons. The data provide a novel mechanistic explanation for synchronized bursting initiation and amplitude modulation through SK channel inhibition. Evidence also shows that synchronized bursting is driven by NMDA (N-methyl-d-aspartate) and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors and that gap junctions do not mediate motoneuron synchronization in this behavior.
Keywords: SK channels; motor output; spinal motoneurons; synchronized bursting.
Copyright © 2017 the American Physiological Society.
Figures





Similar articles
-
A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons.J Neurophysiol. 2004 Jan;91(1):346-57. doi: 10.1152/jn.00062.2003. Epub 2003 Sep 17. J Neurophysiol. 2004. PMID: 13679411
-
Computer simulations of NMDA and non-NMDA receptor-mediated synaptic drive: sensory and supraspinal modulation of neurons and small networks.J Neurophysiol. 1993 Aug;70(2):695-709. doi: 10.1152/jn.1993.70.2.695. J Neurophysiol. 1993. PMID: 8105036
-
Regulation of excitability in tonic firing substantia gelatinosa neurons of the spinal cord by small-conductance Ca(2+)-activated K(+) channels.Neuropharmacology. 2016 Jun;105:15-24. doi: 10.1016/j.neuropharm.2016.01.001. Epub 2016 Jan 8. Neuropharmacology. 2016. PMID: 26777279
-
Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain.Cell Mol Life Sci. 2008 Oct;65(20):3196-217. doi: 10.1007/s00018-008-8216-x. Cell Mol Life Sci. 2008. PMID: 18597044 Free PMC article. Review.
-
Voltage-sensitive ion channels in rhythmic motor systems.Curr Opin Neurobiol. 2002 Dec;12(6):646-51. doi: 10.1016/s0959-4388(02)00377-x. Curr Opin Neurobiol. 2002. PMID: 12490254 Review.
Cited by
-
Episodic rhythmicity is generated by a distributed neural network in the developing mammalian spinal cord.iScience. 2025 Feb 7;28(3):111971. doi: 10.1016/j.isci.2025.111971. eCollection 2025 Mar 21. iScience. 2025. PMID: 40060907 Free PMC article.
-
Dysregulation of persistent inward and outward currents in spinal motoneurons of symptomatic SOD1-G93A mice.J Physiol. 2024 Aug;602(15):3715-3736. doi: 10.1113/JP286032. Epub 2024 Jun 25. J Physiol. 2024. PMID: 38924530 Free PMC article.
-
Muscle Spasms after Spinal Cord Injury Stem from Changes in Motoneuron Excitability and Synaptic Inhibition, Not Synaptic Excitation.J Neurosci. 2024 Jan 3;44(1):e1695232023. doi: 10.1523/JNEUROSCI.1695-23.2023. J Neurosci. 2024. PMID: 37949656 Free PMC article.
-
Modulation of SK channels regulates locomotor alternating bursting activity in the functionally-mature spinal cord.Channels (Austin). 2018 Jan 1;12(1):9-14. doi: 10.1080/19336950.2017.1389825. Epub 2017 Nov 17. Channels (Austin). 2018. PMID: 28991505 Free PMC article.
-
Synaptic disruption and CREB-regulated transcription are restored by K+ channel blockers in ALS.EMBO Mol Med. 2021 Jul 7;13(7):e13131. doi: 10.15252/emmm.202013131. Epub 2021 Jun 14. EMBO Mol Med. 2021. PMID: 34125498 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

