EXO70C2 Is a Key Regulatory Factor for Optimal Tip Growth of Pollen
- PMID: 28356503
- PMCID: PMC5411130
- DOI: 10.1104/pp.16.01282
EXO70C2 Is a Key Regulatory Factor for Optimal Tip Growth of Pollen
Abstract
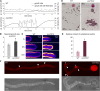
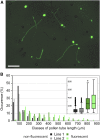
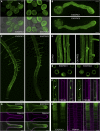
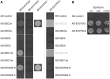
The exocyst, a eukaryotic tethering complex, coregulates targeted exocytosis as an effector of small GTPases in polarized cell growth. In land plants, several exocyst subunits are encoded by double or triple paralogs, culminating in tens of EXO70 paralogs. Out of 23 Arabidopsis thaliana EXO70 isoforms, we analyzed seven isoforms expressed in pollen. Genetic and microscopic analyses of single mutants in EXO70A2, EXO70C1, EXO70C2, EXO70F1, EXO70H3, EXO70H5, and EXO70H6 genes revealed that only a loss-of-function EXO70C2 allele resulted in a significant male-specific transmission defect (segregation 40%:51%:9%) due to aberrant pollen tube growth. Mutant pollen tubes grown in vitro exhibited an enhanced growth rate and a decreased thickness of the tip cell wall, causing tip bursts. However, exo70C2 pollen tubes could frequently recover and restart their speedy elongation, resulting in a repetitive stop-and-go growth dynamics. A pollen-specific depletion of the closest paralog, EXO70C1, using artificial microRNA in the exo70C2 mutant background, resulted in a complete pollen-specific transmission defect, suggesting redundant functions of EXO70C1 and EXO70C2. Both EXO70C1 and EXO70C2, GFP tagged and expressed under the control of their native promoters, localized in the cytoplasm of pollen grains, pollen tubes, and also root trichoblast cells. The expression of EXO70C2-GFP complemented the aberrant growth of exo70C2 pollen tubes. The absent EXO70C2 interactions with core exocyst subunits in the yeast two-hybrid assay, cytoplasmic localization, and genetic effect suggest an unconventional EXO70 function possibly as a regulator of exocytosis outside the exocyst complex. In conclusion, EXO70C2 is a novel factor contributing to the regulation of optimal tip growth of Arabidopsis pollen tubes.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures




Comment in
-
How to Grow a Cell: Fine-Tuning Secretory Activity to Balance Growth and Cell Wall Integrity.Plant Physiol. 2017 May;174(1):3-4. doi: 10.1104/pp.17.00473. Plant Physiol. 2017. PMID: 28461398 Free PMC article.
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Bashline L, Lei L, Li S, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7: 586–600 - PubMed
-
- Chebli Y, Kroeger J, Geitmann A (2013) Transport logistics in pollen tubes. Mol Plant 6: 1037–1052 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

