Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice
- PMID: 28356516
- PMCID: PMC5393247
- DOI: 10.1073/pnas.1619302114
Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice
Abstract
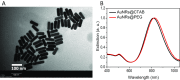
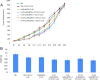
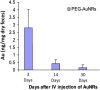
Gold nanorods (AuNRs)-assisted plasmonic photothermal therapy (AuNRs-PPTT) is a promising strategy for combating cancer in which AuNRs absorb near-infrared light and convert it into heat, causing cell death mainly by apoptosis and/or necrosis. Developing a valid PPTT that induces cancer cell apoptosis and avoids necrosis in vivo and exploring its molecular mechanism of action is of great importance. Furthermore, assessment of the long-term fate of the AuNRs after treatment is critical for clinical use. We first optimized the size, surface modification [rifampicin (RF) conjugation], and concentration (2.5 nM) of AuNRs and the PPTT laser power (2 W/cm2) to achieve maximal induction of apoptosis. Second, we studied the potential mechanism of action of AuNRs-PPTT using quantitative proteomic analysis in mouse tumor tissues. Several death pathways were identified, mainly involving apoptosis and cell death by releasing neutrophil extracellular traps (NETs) (NETosis), which were more obvious upon PPTT using RF-conjugated AuNRs (AuNRs@RF) than with polyethylene glycol thiol-conjugated AuNRs. Cytochrome c and p53-related apoptosis mechanisms were identified as contributing to the enhanced effect of PPTT with AuNRs@RF. Furthermore, Pin1 and IL18-related signaling contributed to the observed perturbation of the NETosis pathway by PPTT with AuNRs@RF. Third, we report a 15-month toxicity study that showed no long-term toxicity of AuNRs in vivo. Together, these data demonstrate that our AuNRs-PPTT platform is effective and safe for cancer therapy in mouse models. These findings provide a strong framework for the translation of PPTT to the clinic.
Keywords: apoptosis; gold nanorods; long-term toxicity; plasmonic photothermal therapy; xenograft mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





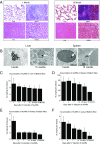
Similar articles
-
Polysarcosine brush stabilized gold nanorods for in vivo near-infrared photothermal tumor therapy.Acta Biomater. 2017 Mar 1;50:534-545. doi: 10.1016/j.actbio.2016.12.050. Epub 2016 Dec 25. Acta Biomater. 2017. PMID: 28027959
-
Photothermal therapy with AuNRs and EGFRmAb-AuNRs inhibits subcutaneous transplantable hypopharyngeal tumors in nude mice.Int J Oncol. 2018 Dec;53(6):2647-2658. doi: 10.3892/ijo.2018.4559. Epub 2018 Sep 12. Int J Oncol. 2018. PMID: 30221691
-
Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy.Biomaterials. 2016 Sep;102:1-8. doi: 10.1016/j.biomaterials.2016.06.017. Epub 2016 Jun 7. Biomaterials. 2016. PMID: 27318931
-
Plasmonic photothermal therapy (PPTT) using gold nanoparticles.Lasers Med Sci. 2008 Jul;23(3):217-28. doi: 10.1007/s10103-007-0470-x. Epub 2007 Aug 3. Lasers Med Sci. 2008. PMID: 17674122 Review.
-
An overview of synthetic strategies and current applications of gold nanorods in cancer treatment.Nanotechnology. 2015 Oct 30;26(43):432001. doi: 10.1088/0957-4484/26/43/432001. Epub 2015 Oct 8. Nanotechnology. 2015. PMID: 26446935 Review.
Cited by
-
Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective.Front Pharmacol. 2021 Apr 22;12:664123. doi: 10.3389/fphar.2021.664123. eCollection 2021. Front Pharmacol. 2021. PMID: 33967809 Free PMC article. Review.
-
Capping gold nanoparticles with albumin to improve their biomedical properties.Int J Nanomedicine. 2019 Aug 9;14:6387-6406. doi: 10.2147/IJN.S210992. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31496693 Free PMC article. Review.
-
Novel Intrapolymerization Doped Manganese-Eumelanin Coordination Nanocomposites with Ultrahigh Relaxivity and Their Application in Tumor Theranostics.Adv Sci (Weinh). 2018 Mar 25;5(7):1800032. doi: 10.1002/advs.201800032. eCollection 2018 Jul. Adv Sci (Weinh). 2018. PMID: 30027037 Free PMC article.
-
Functional Mesoporous Silica Nanocomposites: Biomedical applications and Biosafety.Int J Mol Sci. 2019 Feb 20;20(4):929. doi: 10.3390/ijms20040929. Int J Mol Sci. 2019. PMID: 30791663 Free PMC article. Review.
-
Optimization of endothelial growth factor receptor monoclonal antibody-gold nanorods photothermal therapy for laryngeal squamous cell carcinoma.Bioengineered. 2022 Feb;13(2):3262-3274. doi: 10.1080/21655979.2022.2025517. Bioengineered. 2022. PMID: 35067164 Free PMC article.
References
-
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. - PubMed
-
- Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem C. 2016;120(9):4691–4716.
-
- Steinmetz NF. Review of “Cancer Nanotechnology: Methods and Protocols (Methods in Molecular Biology)” by Stephen R. Grobmyer (Editor), Brij M. Moudgil (Editor) BioMedical Engineering OnLine. 2010;9:55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

