BIOPHYSICAL PROPERTIES OF SUBTHRESHOLD RESONANCE OSCILLATIONS AND SUBTHRESHOLD MEMBRANE OSCILLATIONS IN NEURONS
- PMID: 28356608
- PMCID: PMC5367638
- DOI: 10.1142/S0218339016500285
BIOPHYSICAL PROPERTIES OF SUBTHRESHOLD RESONANCE OSCILLATIONS AND SUBTHRESHOLD MEMBRANE OSCILLATIONS IN NEURONS
Abstract
Subthreshold-level activities in neurons play a crucial role in neuronal oscillations. These small-amplitude oscillations have been suggested to be involved in synaptic plasticity and in determining the frequency of network oscillations. Subthreshold membrane oscillations (STOs) and subthreshold resonance oscillations (SROs) are the main constituents of subthreshold-level activities in neurons. In this study, a general theoretical framework for analyzing the mechanisms underlying STOs and SROs in neurons is presented. Results showed that the resting membrane potential and the hyperpolarization-activated potassium channel (h-channel) affect the subthreshold-level activities in stellate cells. The contribution of h-channel on resonance is attributed to its large time constant, which produces the time lag between Ih and the membrane potential. Conversely, the persistent sodium channels (Nap-channels) only play an amplifying role in these neurons.
Keywords: Biophysical Model; Conductance-Based Model; Equivalent RLC Circuit; Stellate Cells; Subthreshold Membrane Oscillation; Subthreshold Resonance Oscillation.
Conflict of interest statement
Conflict of Interests The authors declare that there is no conflict of interests regarding the publication of this paper
Figures



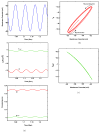
References
-
- Acker CD, Kopell N, White JA. Synchronization of strongly coupled excitatory neurons: Relating network behavior to biophysics. J Comput Neurosci. 2003;15(1):71–90. - PubMed
-
- Wang X-J. Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampalreciprocal loop. J Neurophysiol. 2002;87(2):889–900. - PubMed
-
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338(6213):334–337. - PubMed
-
- Buzsaki G. Two-stage model of memory trace formation: A role for ‘noisy’ brain states. Neuroscience. 1989;31(3):551–570. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
