Inhibition of gap junction intercellular communication is involved in silica nanoparticles-induced H9c2 cardiomyocytes apoptosis via the mitochondrial pathway
- PMID: 28356735
- PMCID: PMC5367603
- DOI: 10.2147/IJN.S127904
Inhibition of gap junction intercellular communication is involved in silica nanoparticles-induced H9c2 cardiomyocytes apoptosis via the mitochondrial pathway
Abstract
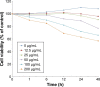
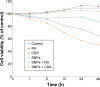
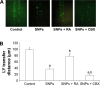
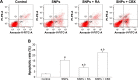
Gap junction intercellular communication (GJIC) between cardiomyocytes is essential for synchronous heart contraction and relies on connexin-containing channels. Connexin 43 (Cx43) is a major component involved in GJIC in heart tissue, and its abnormal expression is closely associated with various cardiac diseases. Silica nanoparticles (SNPs) are known to induce cardiovascular toxicity. However, the mechanisms through which GJIC plays a role in cardiomyocytes apoptosis induced by SNPs remain unknown. The aim of the present study is to determine whether SNPs-decreased GJIC promotes apoptosis in rat cardiomyocytes cell line (H9c2 cells) via the mitochondrial pathway using CCK-8 Kit, scrape-loading dye transfer technique, Annexin V/PI double-staining assays, and Western blot analysis. The results showed that SNPs elicited cytotoxicity in H9c2 cells in a time- and concentration-dependent manner. SNPs also reduced GJIC in H9c2 cells in a concentration-dependent manner through downregulation of Cx43 and upregulation of P-Cx43. Inhibition of gap junctions by gap junction blocker carbenoxolone disodium resulted in decreased survival and increased apoptosis, whereas enhancement of the gap junctions by retinoic acid led to enhanced survival but decreased apoptosis. Furthermore, SNPs-induced apoptosis through the disrupted functional gap junction was correlated with abnormal expressions of the proteins involved in the mitochondrial pathway-related apoptosis such as Bcl-2/Bax, cytochrome C, Caspase-9, and Caspase-3. Taken together, our results provide the first evidence that SNPs-decreased GJIC promotes apoptosis in cardiomyocytes via the mitochondrial pathway. In addition, downregulation of GJIC by SNPs in cardiomyocytes is mediated through downregulation of Cx43 and upregulation of P-Cx43. These results suggest that in rat cardiomyocytes cell line, GJIC plays a protective role in SNPs-induced apoptosis and that GJIC may be one of the targets for SNPs-induced biological effects.
Keywords: cardiotoxicity; cell death; connexin 43; gap junction; silica nanoparticles.
Conflict of interest statement
Disclosure The authors declare that there is no conflict of interest in this work.
Figures


References
-
- Hansen A, Bi P, Nitschke M, et al. Particulate air pollution and cardiorespiratory hospital admissions in a temperate Australian city: a case-crossover analysis. Sci Total Environ. 2012;416:48–52. - PubMed
-
- Du Z, Zhao D, Jing L, et al. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc Toxicol. 2013;13(3):194–207. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

