Second-line afatinib administration in an elderly patient with squamous cell carcinoma
- PMID: 28356747
- PMCID: PMC5367451
- DOI: 10.2147/TCRM.S130816
Second-line afatinib administration in an elderly patient with squamous cell carcinoma
Abstract
Introduction: The majority of cases of lung cancer are still diagnosed at a late stage. At this stage, palliative therapeutic options including nonspecific cytotoxic drugs, targeted therapy, or immunotherapy can be utilized. In 2016, immunotherapy was approved in Europe for squamous cell carcinoma and adenocarcinoma. Moreover, afatinib was also approved as second-line therapy for squamous cell carcinoma.
Case report: This article presents a case of a 76-year-old male with squamous cell carcinoma who received nab-paclitaxel as first-line therapy, and his treatment was switched to the tyrosine kinase inhibitor afatinib (40 mg) after disease progression with left lung atelectasis. After receiving afatinib for only 28 days, the atelectasis resolved. No adverse effects were observed from the afatinib therapy.
Discussion: In this case, afatinib 40 mg proved to be an effective alternative treatment for an elderly patient. Treatment choice should be based on the performance status of the patient, cost-effectiveness, and drug treatment guidelines.
Keywords: EGFR; afatinib; lung cancer.
Conflict of interest statement
Disclosure Paul Zarogoulidis is a member of the advisory board of Boehringer Ingelheim® Greece for Oncology agents. The authors report no other conflicts of interest in this work.
Figures

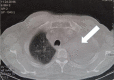
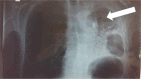
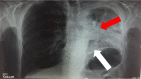
References
-
- Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT. Primary care provider and patient perspectives on lung cancer screening. A qualitative study. Ann Am Thorac Soc. 2016;13(11):1977–1982. - PubMed
-
- Henschke CI, Yip R, Smith JP, et al. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol. 2016;207(6):1176–1184. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

