The US Food and Drug Administration's drug safety recommendations and long-acting beta2-agonist dispensing pattern changes in adult asthma patients: 2003-2012
- PMID: 28356763
- PMCID: PMC5360406
- DOI: 10.2147/JAA.S124395
The US Food and Drug Administration's drug safety recommendations and long-acting beta2-agonist dispensing pattern changes in adult asthma patients: 2003-2012
Abstract
Background: Emerging safety issues associated with long-acting beta2-agonist (LABA) have led to multiple regulatory activities by the US Food and Drug Administration (FDA) since 2003, including Drug Safety Communications (DSCs) in 2010. These DSCs had three specific recommendations for the safe use of LABA products in adult asthma treatment.
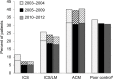
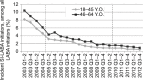
Methods: We examined the initiation of LABA-containing products for adult asthma treatment using an intermittent time series approach in a claims database from 2003 to 2012. We assessed the alignment of dispensing patterns with the following 2010 FDA recommendations: 1) contraindicated use of single-ingredient (SI)-LABA without an asthma controller medication (ACM); 2) a LABA should only be used when asthma is not adequately controlled on inhaled corticosteroids (ICSs) or ACM; and 3) step-down asthma therapy (e.g., discontinue LABA) when asthma control is achieved.
Results: There were 477,922 adults (18-64 years old) dispensed a new LABA during 2003-2012. Among LABA initiators, patients who initiated an SI-LABA and who did "not" have an ACM dispensed on the same date decreased from >9% in 2003 (the initial labeling change) to <2% post 2010 DSCs (p-value <0.0001 in the segmented regression model). The proportion of asthma patients dispensed an ICS in 6 months prior to initiating LABA treatment did not increase. The proportion of patients with longer than 4 months of continuous treatment did not decrease over the study period.
Conclusion: Although the decrease in SI-LABA initiation is consistent with FDA's recommendations, low ICS dispensing before initiating a LABA and LABA continuation practices require further efforts to move toward the recommended safe practices.
Keywords: LABA; US FDA; dispensing pattern; regulatory activities.
Conflict of interest statement
Disclosure All the authors were employees of the US FDA at the time of manuscript completion. The views expressed are those of the authors and not necessarily those of the US FDA. The authors report no other conflicts of interest in this work.
Figures

References
-
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention 2015 (revision) [Accessed January 21, 2017]. Available from: http://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1....
-
- National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. [Accessed Aug 17, 2016]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
-
- FDA [webpage on the Internet] Drugs@FDA Glossary of Terms. [Accessed August 17, 2016]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ucm079436.htm#L.
-
- Food and Drug Administration [webpage on the Internet] Drug Safety Communications. [Accessed August 17, 2016]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm199082.htm.
-
- Perera BJ. Salmeterol multicentre asthma research trial (SMART): interim analysis shows increased risk of asthma related deaths. Ceylon Med J. 2003;48:99. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

