Update on the usage and safety of epinephrine auto-injectors, 2017
- PMID: 28356773
- PMCID: PMC5367766
- DOI: 10.2147/DHPS.S121733
Update on the usage and safety of epinephrine auto-injectors, 2017
Abstract
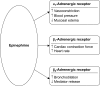
Anaphylaxis is a serious, potentially fatal allergic reaction. Guidelines recommend prompt intramuscular injections of epinephrine as the first-line therapy for anaphylaxis. Delayed epinephrine treatment may cause undesirable clinical outcomes, including death. In the community, epinephrine auto-injectors (EAIs) are commonly used to treat anaphylaxis. This literature review examines several recent concerns regarding the safety of EAIs that may prevent the timely administration of epinephrine. Reports of cardiovascular complications are linked with epinephrine administration, although recent studies suggest that these events are much more commonly associated with intravenous epinephrine rather than with EAIs. Recent studies have also highlighted accidental injections of EAIs in patients' or caregivers' fingers and lacerations associated with the use of EAI in children. However, the data suggest that both accidental injections and lacerations are rare and require limited medical intervention. In addition, patients may receive conflicting information on the safety and efficacy of using expired EAIs. Overall, it is believed that the benefits of using EAIs far outweigh the potential risks of not administering an EAI. Although legitimate safety concerns are associated with EAIs, adverse events are rare. Continued training of medical providers, caregivers, and patients may be beneficial to address these concerns and reduce EAI-associated injuries while ensuring that patients receive necessary medical care.
Keywords: allergy; anaphylaxis; asthma; pediatrics.
Conflict of interest statement
Disclosure Dr Posner has served on the scientific advisory board for Mylan Specialty L.P. and Dr Camargo for Mylan Specialty L.P. and Sanofi-Aventis. Dr Posner and Dr Camargo performed investigator-initiated research funded by Mylan Specialty L.P. The authors report no other conflicts of interest in this work.
Figures



Similar articles
-
Epinephrine Auto-Injectors for Anaphylaxis Treatment in the School Setting: A Discussion Paper.SAGE Open Nurs. 2019 May 23;5:2377960819845246. doi: 10.1177/2377960819845246. eCollection 2019 Jan-Dec. SAGE Open Nurs. 2019. PMID: 33415240 Free PMC article.
-
An update on self-injectable epinephrine.Curr Opin Allergy Clin Immunol. 2013 Aug;13(4):432-7. doi: 10.1097/ACI.0b013e328362b8aa. Curr Opin Allergy Clin Immunol. 2013. PMID: 23743514 Review.
-
Epinephrine, auto-injectors, and anaphylaxis: Challenges of dose, depth, and device.Ann Allergy Asthma Immunol. 2018 Jul;121(1):53-60. doi: 10.1016/j.anai.2018.05.001. Epub 2018 May 7. Ann Allergy Asthma Immunol. 2018. PMID: 29746901 Review.
-
Epinephrine autoinjector prescription patterns for severe anaphylactic patients in Japan: A retrospective analysis of health insurance claims data.Allergol Int. 2020 Jul;69(3):424-428. doi: 10.1016/j.alit.2020.02.008. Epub 2020 Apr 3. Allergol Int. 2020. PMID: 32253115
-
Value of a Second Dose of Epinephrine During Anaphylaxis: A Patient/Caregiver Survey.J Allergy Clin Immunol Pract. 2018 Sep-Oct;6(5):1559-1567. doi: 10.1016/j.jaip.2018.01.019. Epub 2018 Feb 3. J Allergy Clin Immunol Pract. 2018. PMID: 29408441
Cited by
-
Autoinjector - A smart device for emergency cum personal therapy.Saudi Pharm J. 2021 Oct;29(10):1205-1215. doi: 10.1016/j.jsps.2021.09.004. Epub 2021 Sep 20. Saudi Pharm J. 2021. PMID: 34703373 Free PMC article. Review.
-
Accidental Digital Ischemia by an Epinephrine Autoinjector.Cureus. 2023 Mar 20;15(3):e36429. doi: 10.7759/cureus.36429. eCollection 2023 Mar. Cureus. 2023. PMID: 37090392 Free PMC article.
-
Epinephrine delivery via EpiPen® Auto-Injector or manual syringe across participants with a wide range of skin-to-muscle distances.Clin Transl Allergy. 2020 Jun 10;10:21. doi: 10.1186/s13601-020-00326-x. eCollection 2020. Clin Transl Allergy. 2020. PMID: 32528643 Free PMC article.
-
Significant handling errors and education gaps regarding the use of the emergency kit among adult patients with Hymenoptera venom allergy.Allergol Select. 2025 Jan 22;9:1-7. doi: 10.5414/ALX02476E. eCollection 2025. Allergol Select. 2025. PMID: 39881859 Free PMC article.
-
[Paediatric Life Support].Notf Rett Med. 2021;24(4):650-719. doi: 10.1007/s10049-021-00887-9. Epub 2021 Jun 2. Notf Rett Med. 2021. PMID: 34093080 Free PMC article. Review. German.
References
-
- Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–380. - PubMed
-
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69(8):1026–1045. - PubMed
-
- Soar J, Pumphrey R, Cant A, et al. Emergency treatment of anaphylactic reactions – guidelines for healthcare providers. Resuscitation. 2008;77(2):157–169. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

