Quality Assurance in Clinical Chemistry: A Touch of Statistics and A Lot of Common Sense
- PMID: 28356868
- PMCID: PMC5346785
- DOI: 10.1515/jomb-2016-0012
Quality Assurance in Clinical Chemistry: A Touch of Statistics and A Lot of Common Sense
Abstract
Working in laboratories of clinical chemistry, we risk feeling that our personal contribution to quality is small and that statistical models and manufacturers play the major roles. It is seldom sufficiently acknowledged that personal knowledge, skills and common sense are crucial for quality assurance in the interest of patients. The employees, environment and procedures inherent to the laboratory including its interactions with the clients are crucial for the overall result of the total testing chain. As the measurement systems, reagents and procedures are gradually improved, work on the preanalytical, postanalytical and clinical phases is likely to pay the most substantial dividends in accomplishing further quality improvements. This means changing attitudes and behaviour, especially of the users of the laboratory. It requires understanding people and how to engage them in joint improvement processes. We need to use our knowledge and common sense expanded with new skills e.g. from the humanities, management, business and change sciences in order to bring this about together with the users of the laboratory.
Radeći u kliničko-hemijskoj laboratoriji, može nam se učiniti da je naš lični doprinos kvalitetu mali i da glavne uloge pripadaju statističkim modelima i proizvođačima. Retko se u dovoljnoj meri odaje priznanje ličnom znanju, veštinama i zdravoj logici, koji su od presudne važnosti za osiguranje kvaliteta u interesu pacijenata. Zaposleni, okolina i procedure koje se obavljaju u laboratoriji, uključujući njenu interakciju sa klijentima, ključne su za konačni rezultat u ukupnom lancu testiranja. Kako se sistemi merenja, reagensi i procedure postepeno unapređuju, rad na preanalitičkoj, postanalitičkoj i kliničkoj fazi verovatno će se najznačajnije isplatiti u vidu postizanja daljeg napretka u kvalitetu. Ovo znači da treba promeniti stavove i ponašanje, pre svega korisnika laboratorije. Potrebno je razumeti ljude i to na koji način se oni mogu uključiti u zajedničke procese poboljšanja. Moramo upotrebiti svoje znanje i zdravu logiku upotpunjene novim veštinama npr. iz oblasti društvenih nauka, upravljanja, poslovanja i menjati nauke kako bismo ovo postigli zajedno sa korisnicima laboratorije.
Keywords: metrology; postanalytical error; preanalytical error; quality assurance; quality control.
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article
Figures
References
-
- Shewhart WA. Economic control of quality of manufactured product. New York: D. Van Nostrand Company; 1931.
-
- Shewhart WA. Statistical method from the viewpoint of quality control. New York: Dover Publications; 1939.
-
- Small BB. Western Electric Company; 1956. http://www.contesolutions.com/Western_Electric_SQC_Handbook.pdf Available from:
-
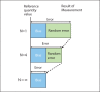
- Levey S, Jennings ER. The use of control charts in the clinical laboratory. Am J Clin Pathol. 1950;20:1059–66. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
