2-Deoxyglucose and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells
- PMID: 28356961
- PMCID: PMC5351389
- DOI: 10.3892/ol.2016.5510
2-Deoxyglucose and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells
Abstract
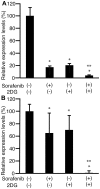
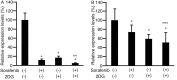
Cancer cells consume more glucose than normal cells, mainly due to their increased rate of glycolysis. 2-Deoxy-d-glucose (2DG) is an analogue of glucose, and sorafenib is a kinase inhibitor and molecular agent used to treat hepatocellular carcinoma (HCC). The present study aimed to demonstrate whether combining 2DG and sorafenib suppresses tumor cell proliferation and motility more effectively than either drug alone. HLF and PLC/PRF/5 HCC cells were incubated with sorafenib with or without 1 µM 2DG, and subjected to a proliferation assay. A scratch assay was then performed to analyze cell motility following the addition of 2DG and sorafenib in combination, and each agent alone. RNA was isolated and subjected to reverse transcription-quantitative polymerase chain reaction to analyze the expression of cyclin D1 and matrix metalloproteinase-9 (MMP9) following the addition of 2DG and sorafenib in combination and each agent alone. Proliferation was markedly suppressed in cells cultured with 1 µM 2DG and 30 µM sorafenib compared with cells cultured with either agent alone (P<0.05). In addition, levels of Cyclin D1 expression decreased in cells exposed to 3 µM sorafenib and 1 µM 2DG compared with cells exposed to 2DG or sorafenib alone (P<0.05). Scratch assay demonstrated that the distance between the growing edge of the cell sheet and the scratched line was shorter in cells cultured with sorafenib and 2DG than in cells cultured with 2DG or sorafenib alone (P<0.05). Levels of MMP9 expression decreased more in cells treated with both sorafenib and 2DG than in cells treated with 2DG or sorafenib alone (P<0.05). Therefore, 2DG and sorafenib in combination suppressed the proliferation and motility of HCC cells more effectively than 2DG or sorafenib alone, and a cancer treatment combining both drugs may be more effective than sorafenib alone.
Keywords: 2-Deoxyglucose; HCC; cyclin D1; matrix metalloproteinase; sorafenib.
Figures


Similar articles
-
Niclosamide suppresses migration of hepatocellular carcinoma cells and downregulates matrix metalloproteinase-9 expression.Oncol Lett. 2015 Dec;10(6):3515-3518. doi: 10.3892/ol.2015.3789. Epub 2015 Oct 9. Oncol Lett. 2015. PMID: 26788160 Free PMC article.
-
Picropodophyllin and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells.Oncol Lett. 2014 Nov;8(5):2023-2026. doi: 10.3892/ol.2014.2484. Epub 2014 Aug 27. Oncol Lett. 2014. PMID: 25289088 Free PMC article.
-
Suppressive effects of 3-bromopyruvate on the proliferation and the motility of hepatocellular carcinoma cells.Oncol Rep. 2016 Jan;35(1):59-63. doi: 10.3892/or.2015.4370. Epub 2015 Nov 2. Oncol Rep. 2016. PMID: 26530887
-
FH535 suppresses the proliferation and motility of hepatocellular carcinoma cells.Int J Oncol. 2016 Jan;48(1):110-4. doi: 10.3892/ijo.2015.3220. Epub 2015 Oct 29. Int J Oncol. 2016. PMID: 26530115
-
Strong enhancement by IGF1-R antagonists of hepatocellular carcinoma cell migration inhibition by Sorafenib and/or vitamin K1.Cell Oncol (Dordr). 2018 Jun;41(3):283-296. doi: 10.1007/s13402-018-0370-z. Epub 2018 Feb 22. Cell Oncol (Dordr). 2018. PMID: 29470830
Cited by
-
Hexokinases II-mediated glycolysis governs susceptibility to crizotinib in ALK-positive non-small cell lung cancer.Thorac Cancer. 2021 Dec;12(23):3184-3193. doi: 10.1111/1759-7714.14184. Epub 2021 Nov 2. Thorac Cancer. 2021. PMID: 34729938 Free PMC article.
-
Redox Homeostasis and Metabolism in Cancer: A Complex Mechanism and Potential Targeted Therapeutics.Int J Mol Sci. 2020 Apr 28;21(9):3100. doi: 10.3390/ijms21093100. Int J Mol Sci. 2020. PMID: 32354000 Free PMC article. Review.
-
Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma.Acta Pharm Sin B. 2022 Feb;12(2):558-580. doi: 10.1016/j.apsb.2021.09.019. Epub 2021 Sep 25. Acta Pharm Sin B. 2022. PMID: 35256934 Free PMC article. Review.
-
Exosomal circRNA: emerging insights into cancer progression and clinical application potential.J Hematol Oncol. 2023 Jun 26;16(1):67. doi: 10.1186/s13045-023-01452-2. J Hematol Oncol. 2023. PMID: 37365670 Free PMC article. Review.
-
Metabolism as a New Avenue for Hepatocellular Carcinoma Therapy.Int J Mol Sci. 2023 Feb 13;24(4):3710. doi: 10.3390/ijms24043710. Int J Mol Sci. 2023. PMID: 36835122 Free PMC article. Review.
References
-
- Katano T, Mizoshita T, Senoo K, Sobue S, Takada H, Sakamoto T, Mochiduki H, Ozeki T, Kato A, Matsunami K, et al. The efficacy of transcatheter arterial embolization as the first-choice treatment after failure of endoscopic hemostasis and endoscopic treatment resistance factors. Dig Endosc. 2012;24:364–369. doi: 10.1111/j.1443-1661.2012.01285.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
