Influence of gap junction intercellular communication composed of connexin 43 on the antineoplastic effect of adriamycin in breast cancer cells
- PMID: 28356970
- PMCID: PMC5351289
- DOI: 10.3892/ol.2016.5471
Influence of gap junction intercellular communication composed of connexin 43 on the antineoplastic effect of adriamycin in breast cancer cells
Abstract
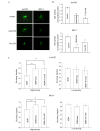
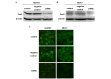
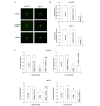
Gap junctions (GJs) serve the principal role in the antineoplastic (cytotoxicity and induced apoptosis) effect of chemical drugs. The aim of the present study was to determine the effect of GJ intercellular communication (GJIC) composed of connexin 43 (Cx43) on adriamycin cytotoxicity in breast cancer cells. Four cell lines (Hs578T, MCF-7, MDA-MB-231 and SK-BR-3) with different degree of malignancy were used in the study. The results of western blotting and immunofluorescence revealed that, in Hs578T and MCF-7 cells, which have a low degree of malignancy, the expression levels of Cx43 and GJIC were higher than those in MDA-MB-231 and SK-BR-3 cells (which have a high degree of malignancy). In Hs578T and MCF-7 cells, where GJ could be formed, the function of GJ was modulated by a pharmacological potentiators [retinoid acid (RA)]/inhibitors [oleamide and 18-α-glycyrrhetinic acid (18-α-GA)] and small interfering RNA (siRNA). In high-density cells (where GJ was formed), enhancement of GJ function by RA increased the cytotoxicity of adriamycin, while inhibition of GJ function by oleamide/18-α-GA and siRNA decreased the cytotoxicity caused by adriamycin. Notably, the modulation of GJ did not affect the survival of cells treated with adriamycin when cells were in low density (no GJ was formed). The present study illustrated the association between GJIC and the antitumor effect of adriamycin in breast cancer cells. The cytotoxicity of adriamycin on breast cancer cells was increased when the function of gap junctions was enhanced.
Keywords: adriamycin; breast cancer; connexin 43; gap junction.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
