Improving data transparency in clinical trials using blockchain smart contracts
- PMID: 28357041
- PMCID: PMC5357027
- DOI: 10.12688/f1000research.9756.1
Improving data transparency in clinical trials using blockchain smart contracts
Abstract
The scientific credibility of findings from clinical trials can be undermined by a range of problems including missing data, endpoint switching, data dredging, and selective publication. Together, these issues have contributed to systematically distorted perceptions regarding the benefits and risks of treatments. While these issues have been well documented and widely discussed within the profession, legislative intervention has seen limited success. Recently, a method was described for using a blockchain to prove the existence of documents describing pre-specified endpoints in clinical trials. Here, we extend the idea by using smart contracts - code, and data, that resides at a specific address in a blockchain, and whose execution is cryptographically validated by the network - to demonstrate how trust in clinical trials can be enforced and data manipulation eliminated. We show that blockchain smart contracts provide a novel technological solution to the data manipulation problem, by acting as trusted administrators and providing an immutable record of trial history.
Keywords: Blockchain; Clinical Trials; Ethereum; Missing Data; Smart Contract.
Conflict of interest statement
Competing interests: The authors are employees of Thomson Reuters.
Figures
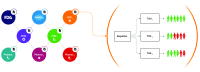
References
-
- Goldacre B: Bad pharma: how drug companies mislead doctors and harm patients. Macmillan.2014. Reference Source
-
- Anderson ML, Chiswell K, Peterson ED, et al. : Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372(11):1031–1039. 10.1056/NEJMsa1409364 - DOI - PMC - PubMed
-
- Nakamoto S: Bitcoin: A peer-to-peer electronic cash system. White Paper. 2008. Reference Source
LinkOut - more resources
Full Text Sources
Other Literature Sources

