Paclitaxel and platinum-based chemotherapy results in transient dyslipidemia in cancer patients
- PMID: 28357107
- PMCID: PMC5351709
- DOI: 10.3892/mco.2016.1107
Paclitaxel and platinum-based chemotherapy results in transient dyslipidemia in cancer patients
Abstract
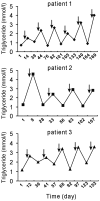
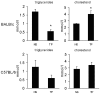
Paclitaxel and cisplatin (TP) are common chemotherapeutic agents extensively used for treating lung and esophageal cancers. The present study reported three patients with transient hypertriglyceridemia (HTG) following TP chemotherapy. Serum triglyceride (TG) levels returned to baseline at chemotherapy intermission. No patient had any history of HTG or any evidence of pancreatitis or other complications of HTG. No regular elevation of any other serum lipids, including cholesterol, high-density lipoprotein and low-density lipoprotein, was observed. However, treatment of mice with TP decreased TG and slightly increased cholesterol. The findings of the present study suggested that TP chemotherapy results in transient dyslipidemia, and physicians must monitor TG levels during chemotherapy to avoid TG-associated complications.
Keywords: chemotherapy; cisplatin; hypertriglyceridemia; paclitaxel.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
