Off-label drug use in hospitalized children: a prospective observational study at Gondar University Referral Hospital, Northwestern Ethiopia
- PMID: 28357130
- PMCID: PMC5368968
- DOI: 10.1002/prp2.304
Off-label drug use in hospitalized children: a prospective observational study at Gondar University Referral Hospital, Northwestern Ethiopia
Abstract
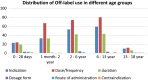
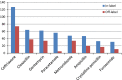
Most of the medications which are currently used for the treatment of childhood diseases are either not licensed or being prescribed outside the terms of the product license (off-label prescribing). This study aimed at determining the extent of unlicensed and off-label drug uses and associated factors in children hospitalized in Gondar University Referral Hospital, Northwest Ethiopia. An institution-based prospective cross-sectional study was employed from April 15 to July 15, 2016. A total of 243 pediatric patients admitted to Gondar university referral hospital were included in the study using simple random sampling method. Data were collected using structured questionnaire, and the data collected were entered and analyzed using Statistical Packages for Social Sciences (SPSS) version 20. From the total of 800 drugs prescribed, 607 (75.8%) were off-label. Off-label medicine use was frequently observed in antimicrobials (60.6%) followed by central nervous system drugs (14.3%). The extent off-label prescribing was highest in age group of 6-13 years (30%). Inappropriate dosing and frequency (42.3%) were the most common reason for off-label medicine use. Having other variables controlled, age group and undergoing surgical procedure remained to be significant predictors of off-label prescribing in the multivariate regression analysis. Implementing evidence-based approach in prescribing by generating more quality literatures on the safety profile and effectiveness of off-label would improve the injudicious use of drugs in pediatric population.
Keywords: Children; Ethiopia; Gondar; off‐label; pediatric.
Figures
Similar articles
-
Off-label and unlicensed drug use in Ayder comprehensive specialized hospital neonatal intensive care unit.Ital J Pediatr. 2020 Apr 3;46(1):41. doi: 10.1186/s13052-020-0809-5. Ital J Pediatr. 2020. PMID: 32245504 Free PMC article.
-
Nonadherence to Antiepileptic Medications and Its Determinants among Epileptic Patients at the University of Gondar Referral Hospital, Gondar, Ethiopia, 2019: An Institutional-Based Cross-Sectional Study.Neurol Res Int. 2020 Oct 28;2020:8886828. doi: 10.1155/2020/8886828. eCollection 2020. Neurol Res Int. 2020. PMID: 33194230 Free PMC article.
-
[Use of unlicensed and off-label drugs in hospitalized children].Harefuah. 1999 Dec 1;137(11):532-4, 591. Harefuah. 1999. PMID: 10959365 Hebrew.
-
Extent, reasons and consequences of off-labeled and unlicensed drug prescription in hospitalized children: a narrative review.World J Pediatr. 2021 Aug;17(4):341-354. doi: 10.1007/s12519-021-00430-3. Epub 2021 Jun 2. World J Pediatr. 2021. PMID: 34080130 Review.
-
Off-label and unlicensed drug prescribing in three paediatric wards in Finland and review of the international literature.J Clin Pharm Ther. 2009 Jun;34(3):277-87. doi: 10.1111/j.1365-2710.2008.01005.x. J Clin Pharm Ther. 2009. PMID: 19650250 Review.
Cited by
-
Ursodeoxycholic acid use is associated with significant risk of morbidity and mortality in infants with cholestasis: A strobe compliant study.Medicine (Baltimore). 2020 Feb;99(7):e18730. doi: 10.1097/MD.0000000000018730. Medicine (Baltimore). 2020. PMID: 32049781 Free PMC article.
-
Off‑label and unapproved pediatric drug utilization: A meta‑analysis.Exp Ther Med. 2024 Aug 30;28(5):412. doi: 10.3892/etm.2024.12701. eCollection 2024 Nov. Exp Ther Med. 2024. PMID: 39268368 Free PMC article.
-
HPLC-(Q)-TOF-MS-Based Study of Plasma Metabolic Profile Differences Associated with Age in Pediatric Population Using an Animal Model.Metabolites. 2022 Aug 11;12(8):739. doi: 10.3390/metabo12080739. Metabolites. 2022. PMID: 36005611 Free PMC article.
-
Off-label and unlicensed drug use in Ayder comprehensive specialized hospital neonatal intensive care unit.Ital J Pediatr. 2020 Apr 3;46(1):41. doi: 10.1186/s13052-020-0809-5. Ital J Pediatr. 2020. PMID: 32245504 Free PMC article.
-
"Off-Label" Medicine Use In Burned Children: Three-Year Retrospective Study.Ann Burns Fire Disasters. 2021 Mar 31;34(1):18-25. Ann Burns Fire Disasters. 2021. PMID: 34054383 Free PMC article.
References
-
- Bavdekar SB, Sadawarte PA, Gogtay NJ, Jain SS, Jadhav S (2009). Off‐label drug use in a Paediatric Intensive Care Unit. Indian J Pediatr 76: 1113–1118. - PubMed
-
- Clavenna A, Bonati M (2009). Drug prescriptions to outpatient children: a review of the literature. Eur J Clin Pharmacol 65: 749–755. - PubMed
-
- Conroy S, Peden V (2001). Unlicensed and off label analgesic use in pediatrics pain management. Pediatr Anesth 11: 431–436. - PubMed
-
- Cuzzolin L, Atzei A, Fanos V (2006). Off‐label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 5: 703–718. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous