Development and Pretesting of a Questionnaire to Assess Patient Experiences and Satisfaction with Medications (PESaM Questionnaire)
- PMID: 28357591
- PMCID: PMC5605609
- DOI: 10.1007/s40271-017-0234-z
Development and Pretesting of a Questionnaire to Assess Patient Experiences and Satisfaction with Medications (PESaM Questionnaire)
Abstract
Background: The aim of this study was to develop, together with the Lung Foundation Netherlands and Dutch Kidney Patients Association, patients and clinicians, a measure to evaluate patient experiences with the orphan drugs pirfenidone (for idiopathic pulmonary fibrosis [IPF]) and eculizumab (for atypical haemolytic uraemic syndrome [aHUS]), as well as a generic measure of patient experiences and satisfaction with medications.
Methods: Development of the Patient Experiences and Satisfaction with Medications (PESaM) questionnaire consisted of four phases: literature review (phase I); focus groups and individual patient interviews (phase II); item generation (phase III); and face and content validity testing (phase IV). Literature review aimed to identify existing disease-specific and generic patient experience measures to provide guidance on the domains of medication use relevant to patients, the number of items and type of response categories, and to generate an initial pool of items. Subsequent focus groups and patient interviews were conducted to gain insight into the perceived effectiveness of the therapies, the burden of side effects, and how the medication impacted on a patient's daily life. Focus groups and interviews were recorded and transcribed verbatim. Coding was carried out by highlighting passages in the text and assigning each passage a code representing the following predefined categories: (1) perceived effectiveness; (2) side effects; (3) ease of use; and (4) impact of medication. Using data from phase I and II, a panel of experts selected items relevant for inclusion in the questionnaire. Individual patient interviews with IPF and aHUS patients (n = 18), using a retrospective verbal probing technique, were conducted to assess face validity, time needed to fill out the questionnaire, and content validity.
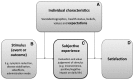
Results: The PESaM questionnaire that was developed consisted of two disease-specific modules that assessed patient experiences with pirfenidone for the treatment of IPF, and eculizumab for the treatment of aHUS, a generic module, applicable to any medication, and a module to assess patient expectations. Review of the literature identified multiple disease- or medication-specific questionnaires and two generic patient satisfaction questionnaires. Common domains across most questionnaires were effectiveness, side effects, ease of use and overall satisfaction. Patient interviews revealed the social impact (e.g. unable to go outside) of side effects such as photosensitivity associated with pirfenidone and the risk of infection associated with eculizumab. Each PESaM module focuses on patients' perceived effectiveness of the medication, side effects, and ease of use, and the impact these aspects have on physical and emotional health and daily life. The generic module additionally includes items related to satisfaction with the medication. Individual interviews with patients in phase IV confirmed, in general, that questions and response options of the modules were clear and content validity was good. The mean time to complete the modules ranged from 6 min for the disease-specific (aHUS) module to 9 min for the generic module.
Conclusions: We developed the PESaM questionnaire to quantitatively assess patient experiences and satisfaction with medications. A validation study is currently underway to examine the psychometric properties of the PESaM questionnaire.
Keywords: Eculizumab; Focus Group; Idiopathic Pulmonary Fibrosis; Orphan Drug; Patient Experience.
Conflict of interest statement
Conflict of interest
Merel Kimman, Adrienne Rotteveel, Marlies Wijsenbeek, Rémy Mostard, Nelleke Tak, Xana van Jaarsveld, Marjolein Storm, Kioa Wijnsma, Marielle Gelens, Nicole van de Kar, Jack Wetzels and Carmen Dirksen have no conflicts of interest, including nonfinancial, that are directly relevant to the content of this article.
Funding
This project was funded by The Federation of Patients and Consumer Organisations in The Netherlands (NPCF), Lung Foundation Netherlands, and The Netherlands Organisation for Health Research and Development (ZonMw). We thank Ms Jeanine van der Giessen (M.Sc.), health literacy specialist, University Medical Center Utrecht, for reviewing the questionnaires. We also gratefully acknowledge the support of Ms. Mirjam van Manen (M.Sc.), Ph.D. candidate at the Department of Respiratory Medicine of the Erasmus Medical Center in Rotterdam.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

