A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies
- PMID: 28357727
- PMCID: PMC5447332
- DOI: 10.1007/s11523-017-0482-9
A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies
Abstract
Background: The combination of everolimus and the imidazoquinoline derivative, BEZ235 (dactolisib), a dual PI3K/mTOR inhibitor, demonstrated synergy in a preclinical model.
Objective: To establish clinical feasibility, a phase Ib dose-escalation trial investigating safety and pharmacokinetics of this combination in patients with advanced tumors was performed.
Patients and methods: BEZ235 was orally administered daily in escalating doses of 200, 400, and 800 mg along with everolimus at 2.5 mg daily in 28-day cycles. Nineteen patients were enrolled. Adverse events and tumor responses were evaluated using CTCAE v4.0 and RECIST 1.1, respectively. Pharmacokinetic analyses were performed.
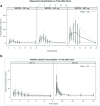
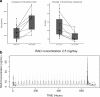
Results: Common toxicities observed included fatigue, diarrhea, nausea, mucositis, and elevated liver enzymes. No confirmed responses were observed. BEZ235 pharmacokinetics exhibited dose-proportional increases in Cmax and AUC0-24 over the three doses, with high inter-individual variability. Non-compartmental and population pharmacokinetic-based simulations indicated significant increases in everolimus Cmax and AUC0-24 on day 28 and decreased clearance to 13.41 L/hr.
Conclusions: The combination of BEZ235 and everolimus demonstrated limited efficacy and tolerance. BEZ235 systemic exposure increased in a dose-proportional manner while oral bioavailability was quite low, which may be related to gastrointestinal-specific toxicity. The changes in steady-state pharmacokinetics of everolimus with BEZ235 highlight potential drug-drug interactions when these two drugs are administered together. Clinicaltrials.gov: NCT01508104.
Conflict of interest statement
Funding
The investigational agent, dactolisib (BEZ 235) used in this study, and funding for the pharmacokinetic analysis of patient samples was provided by Novartis Oncology (East Hanover, NJ, USA). Trisha Wise-Draper is supported by the clinical scientist training program at the University of Cincinnati (UC). Hala Elnakat Thomas is supported by a faculty pilot project grant by the Department of Internal Medicine at UC and a just-in-time award by the UC Cancer Center. We would also like to thank the Lcs Foundation for their support.
Conflict of Interest
Nagal Abdel Karim has received payment for lectures on everolimus for FDA-approved indications. Sara Kozma was an employee at the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland, which is affiliated with the Novartis Institute for Biomedical Research, from 1986 to 2003. George Thomas was employed as a scientific consultant by Novartis Oncology from 2000–2010. Olivier Rixe has received grants from Novartis through the University of Cincinnati to support the clinical trial and correlative studies. All other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

