CXCR3+ monocytes/macrophages are required for establishment of pulmonary metastases
- PMID: 28358049
- PMCID: PMC5372355
- DOI: 10.1038/srep45593
CXCR3+ monocytes/macrophages are required for establishment of pulmonary metastases
Abstract
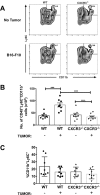
We present a new foundational role for CXCR3+ monocytes/macrophages in the process of tumor engraftment in the lung. CXCR3 is associated with monocytic and lymphocytic infiltration of inflamed or tumor-bearing lung. Although the requirement for tumor-expressed CXCR3 in metastatic engraftment has been demonstrated, the role of monocyte-expressed CXCR3 had not been appreciated. In a murine model of metastatic-like melanoma, engraftment was coordinate with CXCR3+ monocyte/macrophage accumulation in the lungs and was sensitive to pharmacologic inhibition of CXCR3 signaling. Tumor engraftment to lung was impaired in CXCR3-/- mice, and transient reconstitution with circulating CXCR3-replete monocytes was sufficient to restore engraftment. These data illustrate the paradoxical pro-tumor role for CXCR3 in lung immunobiology wherein the CXCR3 axis drives both the anti-tumor effector cell chemoattraction and pro-tumor infiltration of the lungs and suggests a potential therapeutic target for lung-tropic metastasizing cancers.
Figures





Similar articles
-
Melanoma Induces, and Adenosine Suppresses, CXCR3-Cognate Chemokine Production and T-cell Infiltration of Lungs Bearing Metastatic-like Disease.Cancer Immunol Res. 2015 Aug;3(8):956-67. doi: 10.1158/2326-6066.CIR-15-0015. Epub 2015 Jun 5. Cancer Immunol Res. 2015. PMID: 26048575 Free PMC article.
-
Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis.Cell Rep. 2019 Aug 13;28(7):1785-1798.e6. doi: 10.1016/j.celrep.2019.07.033. Cell Rep. 2019. PMID: 31412247
-
CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses.J Exp Med. 2010 Aug 30;207(9):1951-66. doi: 10.1084/jem.20100098. Epub 2010 Aug 23. J Exp Med. 2010. PMID: 20733031 Free PMC article.
-
Lung Macrophages: Multifunctional Regulator Cells for Metastatic Cells.Int J Mol Sci. 2018 Dec 29;20(1):116. doi: 10.3390/ijms20010116. Int J Mol Sci. 2018. PMID: 30597969 Free PMC article. Review.
-
Contribution of CXCR3-mediated signaling in the metastatic cascade of solid malignancies.Biochim Biophys Acta Rev Cancer. 2021 Dec;1876(2):188628. doi: 10.1016/j.bbcan.2021.188628. Epub 2021 Sep 22. Biochim Biophys Acta Rev Cancer. 2021. PMID: 34560199 Free PMC article. Review.
Cited by
-
CD33 Expression on Peripheral Blood Monocytes Predicts Efficacy of Anti-PD-1 Immunotherapy Against Non-Small Cell Lung Cancer.Front Immunol. 2022 Apr 14;13:842653. doi: 10.3389/fimmu.2022.842653. eCollection 2022. Front Immunol. 2022. PMID: 35493454 Free PMC article.
-
Harnessing innate lung anti-cancer effector functions with a novel bacterial-derived immunotherapy.Oncoimmunology. 2017 Nov 27;7(3):e1398875. doi: 10.1080/2162402X.2017.1398875. eCollection 2018. Oncoimmunology. 2017. PMID: 29399400 Free PMC article.
-
The Expression of Adenosine A2B Receptor on Antigen-Presenting Cells Suppresses CD8+ T-cell Responses and Promotes Tumor Growth.Cancer Immunol Res. 2020 Aug;8(8):1064-1074. doi: 10.1158/2326-6066.CIR-19-0833. Epub 2020 May 7. Cancer Immunol Res. 2020. PMID: 32381524 Free PMC article.
-
Reactive myelopoiesis and FX-expressing macrophages triggered by chemotherapy promote cancer lung metastasis.JCI Insight. 2023 May 8;8(9):e167499. doi: 10.1172/jci.insight.167499. JCI Insight. 2023. PMID: 36976637 Free PMC article.
-
Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy.Cancer Cell. 2024 Jan 8;42(1):35-51.e8. doi: 10.1016/j.ccell.2023.11.011. Epub 2023 Dec 21. Cancer Cell. 2024. PMID: 38134936 Free PMC article.
References
-
- Hiratsuka S., Watanabe A., Aburatani H. & Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–75 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

