Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit
- PMID: 28358077
- PMCID: PMC5492514
- DOI: 10.1038/nature21692
Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit
Abstract
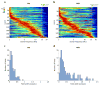
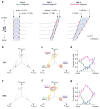
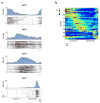
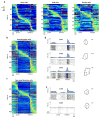
During spatial navigation, neural activity in the hippocampus and the medial entorhinal cortex (MEC) is correlated to navigational variables such as location, head direction, speed, and proximity to boundaries. These activity patterns are thought to provide a map-like representation of physical space. However, the hippocampal-entorhinal circuit is involved not only in spatial navigation, but also in a variety of memory-guided behaviours. The relationship between this general function and the specialized spatial activity patterns is unclear. A conceptual framework reconciling these views is that spatial representation is just one example of a more general mechanism for encoding continuous, task-relevant variables. Here we tested this idea by recording from hippocampal and entorhinal neurons during a task that required rats to use a joystick to manipulate sound along a continuous frequency axis. We found neural representation of the entire behavioural task, including activity that formed discrete firing fields at particular sound frequencies. Neurons involved in this representation overlapped with the known spatial cell types in the circuit, such as place cells and grid cells. These results suggest that common circuit mechanisms in the hippocampal-entorhinal system are used to represent diverse behavioural tasks, possibly supporting cognitive processes beyond spatial navigation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

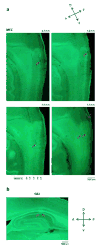
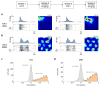
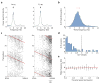
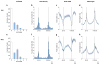




Comment in
-
Neuroscience: Auditory landscape on the cognitive map.Nature. 2017 Mar 29;543(7647):631-632. doi: 10.1038/543631a. Nature. 2017. PMID: 28358066 No abstract available.
-
Navigation: Sounds like non-spatial navigation.Nat Rev Neurosci. 2017 Jun;18(6):322-323. doi: 10.1038/nrn.2017.53. Epub 2017 Apr 24. Nat Rev Neurosci. 2017. PMID: 28435164 No abstract available.
References
-
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain research. 1971;34:171–175. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

