Tubular Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells Generate Pulse Pressure In Vivo
- PMID: 28358136
- PMCID: PMC5371992
- DOI: 10.1038/srep45499
Tubular Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells Generate Pulse Pressure In Vivo
Abstract
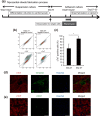
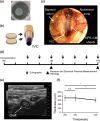
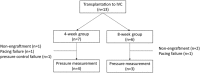
Human induced pluripotent stem (iPS) cell-derived cardiac cells provide the possibility to fabricate cardiac tissues for transplantation. However, it remains unclear human bioengineered cardiac tissues function as a functional pump in vivo. Human iPS cells induced to cardiomyocytes in suspension were cultured on temperature-responsive dishes to fabricate cardiac cell sheets. Two pairs of triple-layered sheets were transplanted to wrap around the inferior vena cava (IVC) of nude rats. At 4 weeks after transplantation, inner pressure changes in the IVC were synchronized with electrical activations of the graft. Under 80 pulses per minute electrical stimulation, the inner pressure changes at 8 weeks increased to 9.1 ± 3.2 mmHg, which were accompanied by increases in the baseline inner pressure of the IVC. Immunohistochemical analysis revealed that 0.5-mm-thick cardiac troponin T-positive cardiac tissues, which contained abundant human mitochondria, were clearly engrafted lamellar around the IVC and surrounded by von Willebrand factor-positive capillary vessels. The mRNA expression of several contractile proteins in cardiac tissues at 8 weeks in vivo was significantly upregulated compared with those at 4 weeks. We succeeded in generating pulse pressure by tubular human cardiac tissues in vivo. This technology might lead to the development of a bioengineered heart assist pump.
Conflict of interest statement
Tatsuya Shimizu is a stakeholder in CellSeed Inc. Tokyo Women’s Medical University received research funds from CellSeed Inc. Tatsuya Shimizu and Katsuhisa Matsuura are inventors of bioreactor systems. Tokyo Women’s Medical University, Tatsuya Shimizu and Katsuhisa Matsuura received a licence fee from ABLE Corporation. The other authors have no conflicts.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

