Enantioselective Diels-Alder-lactamization organocascades employing a furan-based diene
- PMID: 28358148
- PMCID: PMC5522755
- DOI: 10.1039/c6ob02738e
Enantioselective Diels-Alder-lactamization organocascades employing a furan-based diene
Abstract
α,β-Unsaturated acylammonium salts are useful dienophiles enabling highly enantioselective and stereodivergent Diels-Alder-initiated organocascades with furan-based dienes. Complex polycyclic systems can thus be obtained from readily prepared dienes, commodity acid chlorides, and a chiral isothiourea organocatalyst under mild conditions. We describe the use of furan-based dienes bearing pendant sulfonamides leading to the generation of oxa-bridged, trans-fused tricyclic γ-lactams. This process constitutes the first highly enantio- and diastereoselective, organocatalytic Diels-Alder cycloadditions with these typically problematic dienes due to their reversibility. Computational studies suggest that the high diastereoselectivity with these furan dienes may be due to a reversible Diels-Alder cycloaddition for the endo adducts. In addition, the utility of this methodology is demonstrated through a concise approach to a core structure with similarity to the natural product isatisine A and a nonpeptidyl ghrelin-receptor inverse agonist.
Figures




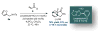
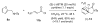
References
-
- Warrener RN, Margetic D, Sun G. Tetrahedron Lett. 2001;42:4263.
- Zubkov FI, Zaytsev VP, Nikitina EV, Khrustalev VN, Gozun SV, Boltukhina EV, Varlamov AV. Tetrahedron. 2011;67:9148.
- Froidevaux V, Borne M, Laborbe E, Auvergne R, Gandini A, Boutevin B. RSC Adv. 2015;5:37742.
- Lacerda TM, Carvalhoc AJF, Gandini A. RSC Adv. 2016;6:45696.
-
- Evans DA, Barnes DM. Tetrahedron Lett. 1997;38:57.
-
- Evans DA, Barnes DM, Johnson JS, Lectka T, von Matt P, Miller SJ, Murry JA, Norcross RD, Shaughnessy EA, Campos KR. J Am Chem Soc. 1999;121:7582.
-
- Ryu DH, Kim KH, Sim JY, Corey EJ. Tetrahedron Lett. 2007;48:5735.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

