New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets
- PMID: 28358273
- PMCID: PMC5446063
- DOI: 10.1080/15548627.2017.1290751
New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets
Abstract
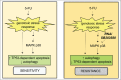
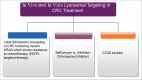
Colorectal cancer (CRC), despite numerous therapeutic and screening attempts, still remains a major life-threatening malignancy. CRC etiology entails both genetic and environmental factors. Macroautophagy/autophagy and the unfolded protein response (UPR) are fundamental mechanisms involved in the regulation of cellular responses to environmental and genetic stresses. Both pathways are interconnected and regulate cellular responses to apoptotic stimuli. In this review, we address the epidemiology and risk factors of CRC, including genetic mutations leading to the occurrence of the disease. Next, we discuss mutations of genes related to autophagy and the UPR in CRC. Then, we discuss how autophagy and the UPR are involved in the regulation of CRC and how they associate with obesity and inflammatory responses in CRC. Finally, we provide perspectives for the modulation of autophagy and the UPR as new therapeutic options for CRC treatment.
Keywords: Beclin 1; ER-stress; GRP78; autophagy; cancer therapy; colorectal cancer; unfolded protein response.
Figures












References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107 - DOI - PubMed
-
- Canada CCSNCIo Canadian Cancer Statistics 2008. Toronto: Canadian Cancer Society, 2008
-
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009; 59:366-78; PMID:19897840; http://dx.doi.org/10.3322/caac.20038 - DOI - PubMed
-
- Saudi Moh Health awareness center: “Cancer incidence in the Kingdom, Compared to the global incidence, Is still low. Saudi Arabia: Ministry of health, 2013
-
- Dolatkhah R, Somi MH, Bonyadi MJ, Asvadi Kermani I, Farassati F, Dastgiri S. Colorectal cancer in iran: Molecular epidemiology and screening strategies. J Cancer Epidemiol 2015; 2015:643020; PMID:25685149; http://dx.doi.org/10.1155/2015/643020 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
