Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: in UK primary care: a real-world, retrospective, observational
- PMID: 28358398
- PMCID: PMC5375386
- DOI: 10.1038/npjpcrm.2016.2
Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: in UK primary care: a real-world, retrospective, observational
Erratum in
-
Erratum: Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: a real-world study.NPJ Prim Care Respir Med. 2017 Jun 8;27:17004. doi: 10.1038/npjpcrm.2017.4. NPJ Prim Care Respir Med. 2017. PMID: 28631719 Free PMC article.
Abstract
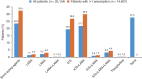
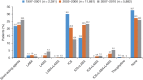
Prescribing patterns in chronic obstructive pulmonary disease (COPD) are often inconsistent with published guidelines. This retrospective, observational study utilised data from the Optimum Patient Care Research Database to examine the changes in COPD prescribing patterns over time and to identify predictors of physician treatment choice for patients newly diagnosed with COPD. Initial therapy was defined as the treatment(s) prescribed at or within 1 year before COPD diagnosis. Changes over time were assessed in three cohorts based on the date of diagnosis: (1) 1997-2001; (2) 2002-2006; and (3) 2007-2010. Factors affecting the odds of being prescribed any initial therapy or any initial maintenance therapy were identified by univariable and multivariable logistic regression. The analysis included 20,154 patients, 45% of whom were prescribed an initial regimen containing an inhaled corticosteroid (ICS), whereas 28% received no initial pharmacological treatment. Prescribing of ICS monotherapy decreased over time, as did the proportion of patients receiving no therapy at or within 1 year before diagnosis. Comorbid asthma, a high exacerbation rate, increased symptoms and poor lung function each increased the likelihood of being prescribed any initial therapy or initial maintenance therapy; comorbid asthma and an annual rate of ⩾3 exacerbations were the strongest predictors. In conclusion, our analyses revealed major differences between actual prescribing behaviour and guideline recommendations for patients with newly diagnosed COPD, with many patients receiving no treatment and large numbers of patients receiving ICS-containing regimens. Predictors of initial therapy were identified.
Conflict of interest statement
KG-J has acted as a consultant for, and spoken on behalf of, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma/Napp, Novartis and Teva. GB has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, Novartis, Pfizer and UCB; he is a member of advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis. RJ has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Health Intelligence; grants, personal fees and non-financial support from Novartis; and personal fees and non-financial support from Mundipharma/Napp. MM has received speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Grifols, Menarini, Novartis and Pfizer, and consulting fees from Almirall, Boehringer Ingelheim, CSL Behring, Gebro Pharma, GlaxoSmithKline, Grifols, MedImmune, Novartis and Pfizer. MB was an employee of Novartis at study initiation. Currently, he is an employee of Boehringer Ingelheim GmbH (Germany). RS, AR and ED are employees of Research in Real-Life, which has conducted paid research in respiratory disease on behalf of the following organisations: Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva. DLK is an employee of Novartis Pharma AG (Basel, Switzerland). DP discloses the following. Advisory board membership: Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis and Teva. Consultancy: Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer and Teva. Grants/grants pending: Aerocrine, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, UK National Health Service and Zentiva. Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda and Teva. Payment for manuscript preparation: Mundipharma and Teva. Patents (planned, pending or issued): AKL Ltd. Payment for the development of educational materials: GlaxoSmithKline and Novartis. Stock/stock options: shares in AKL Ltd, which produces phytopharmaceuticals, and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care. Payment for travel/accomodation/meeting expenses: Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva. Funding for patient enrolment or completion of research: Almirall, Chiesi, Teva and Zentiva. Peer reviewer for grant committees: Efficacy and Mechanism Evaluation programme (2012), HTA (2014) and Medical Research Council (2014). Unrestricted funding for investigator-initiated studies: Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva.
Figures
References
-
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir. Med. 2003; 97 (Suppl C): S71–S79. - PubMed
-
- Roche, N. et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir. Med. 1, e29–e30 (2013). - PubMed
-
- Travers, J. et al. External validity of randomized controlled trials in COPD. Respir. Med. 101, 1313–1320 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

