Differential autophagic effects of vital dyes in retinal pigment epithelial ARPE-19 and photoreceptor 661W cells
- PMID: 28358857
- PMCID: PMC5373602
- DOI: 10.1371/journal.pone.0174736
Differential autophagic effects of vital dyes in retinal pigment epithelial ARPE-19 and photoreceptor 661W cells
Abstract
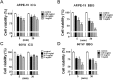
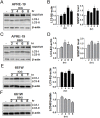
Indocyanine green (ICG) and brilliant blue G (BBG) are commonly used vital dyes to remove internal limiting membrane (ILM) in vitreoretinal surgery. The vital dyes have shown cytotoxic effects in ocular cells. Autophagy is a stress responsive pathway for either protecting cells or promoting cell death. However, the role of autophagy in ocular cells in response to the vital dyes remains unknown. In this study, we found that ICG and BBG reduced cell viability in both human retinal pigment epithelial ARPE-19 and mouse photoreceptor 661W cells. ICG and BBG induced lipidated GFP-LC3-II and LC3-II in ARPE-19 and 661W cells. Combination treatment with the autophagy inhibitor chloroquine indicated that ICG and BBG reduced autophagic flux in ARPE-19 cells, whereas the vital dyes induced autophagic flux in 661W cells. Moreover, genetic and pharmacological ablation of autophagy enhanced vital dyes-induced cytotoxicity in ocular cells. Dietary supplements, including resveratrol, lutein, and CoQ10, induced autophagy and diminished the cytotoxic effects of ICG and BBG in ocular cells. These results suggest that autophagy may protect ARPE-19 and 661W cells from vital dyes-induced damage.
Conflict of interest statement
Figures




Similar articles
-
Metformin and rapamycin protect cells from vital dye-induced damage in retinal pigment epithelial cells and in vivo.Graefes Arch Clin Exp Ophthalmol. 2020 Mar;258(3):557-564. doi: 10.1007/s00417-019-04548-z. Epub 2020 Jan 14. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31938854
-
Comparative in vitro safety analysis of dyes for chromovitrectomy: indocyanine green, brilliant blue green, bromophenol blue, and infracyanine green.Retina. 2011 Jun;31(6):1128-36. doi: 10.1097/IAE.0b013e3181fe543a. Retina. 2011. PMID: 21394068
-
Phototoxicity of indocyanine green and Brilliant Blue G under continuous fluorescent illumination on cultured human retinal pigment epithelial cells.Invest Ophthalmol Vis Sci. 2012 Oct 25;53(11):7389-94. doi: 10.1167/iovs.12-10754. Invest Ophthalmol Vis Sci. 2012. PMID: 23033383
-
Vital dyes for chromovitrectomy.Curr Opin Ophthalmol. 2007 May;18(3):179-87. doi: 10.1097/ICU.0b013e32811080b5. Curr Opin Ophthalmol. 2007. PMID: 17435423 Review.
-
Safety parameters for indocyanine green in vitreoretinal surgery.Dev Ophthalmol. 2008;42:43-68. doi: 10.1159/000138924. Dev Ophthalmol. 2008. PMID: 18535380 Review.
Cited by
-
Efficacy of different doses of dye-assisted internal limiting membrane peeling in idiopathic macular hole: a systematic review and network meta-analysis.Int Ophthalmol. 2021 Mar;41(3):1129-1140. doi: 10.1007/s10792-020-01656-2. Epub 2021 Jan 3. Int Ophthalmol. 2021. PMID: 33392941
-
A Re-Appraisal of Pathogenic Mechanisms Bridging Wet and Dry Age-Related Macular Degeneration Leads to Reconsider a Role for Phytochemicals.Int J Mol Sci. 2020 Aug 3;21(15):5563. doi: 10.3390/ijms21155563. Int J Mol Sci. 2020. PMID: 32756487 Free PMC article. Review.
-
ERBB2-modulated ATG4B and autophagic cell death in human ARPE19 during oxidative stress.PLoS One. 2019 Mar 14;14(3):e0213932. doi: 10.1371/journal.pone.0213932. eCollection 2019. PLoS One. 2019. PMID: 30870514 Free PMC article.
-
Internal limiting membrane peeling with different dyes in the surgery of idiopathic macular hole: a systematic review of literature and network Meta-analysis.Int J Ophthalmol. 2019 Dec 18;12(12):1917-1928. doi: 10.18240/ijo.2019.12.15. eCollection 2019. Int J Ophthalmol. 2019. PMID: 31850178 Free PMC article.
-
Metformin and rapamycin protect cells from vital dye-induced damage in retinal pigment epithelial cells and in vivo.Graefes Arch Clin Exp Ophthalmol. 2020 Mar;258(3):557-564. doi: 10.1007/s00417-019-04548-z. Epub 2020 Jan 14. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31938854
References
-
- Smiddy WE. The current status of macular hole surgery. Bull Soc Belge Ophtalmol. 1996;262:31–42. Epub 1996/01/01. - PubMed
-
- Yooh HS, Brooks HL Jr., Capone A Jr., L'Hernault NL, Grossniklaus HE. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996;122(1):67–75. - PubMed
-
- Maia M, Haller JA, Pieramici DJ, Margalit E, de Juan E Jr., Farah ME, et al. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina. 2004;24(1):157–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

